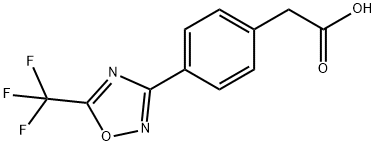
D0131 synthesis
- Product Name:D0131
- CAS Number:1418293-44-3
- Molecular formula:C11H7F3N2O3
- Molecular Weight:272.18
Yield:1418293-44-3 17.8 g
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20;
Steps:
15.A Step A:
Preparation of 4-[5-(trifluoromethyl)-1,2,4-oxadiazol-3-yl]benzeneacetic acid
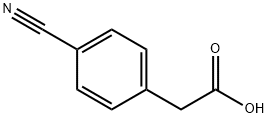
A mixture of 4-cyanobenzeneacetic acid (25.0 g, 155 mmol) and hydroxylamine (50% aqueous solution, 23.8 mL, 780 mmol) in ethanol (500 mL) was heated at reflux overnight.
The reaction mixture was concentrated under reduced pressure and the resulting solid was dried in a vacuum oven overnight.
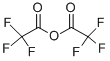
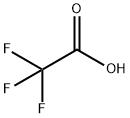
The solid (i.e. the intermediate compound 4-[(hydroxyamino)iminomethyl]benzeneacetic acid) was suspended in tetrahydrofuran (500 mL) and cooled to 0° C., and then trifluoroacetic anhydride (48 mL, 340 mmol) and triethylamine (47 mL, 340 mmol) were added.
The reaction mixture was stirred at room temperature overnight, and then concentrated under reduced.
The resulting material was partitioned between water and dichloromethane.
The organic layer was separated, dried over sodium sulfate, filtered, and concentrated onto Celite (diatomaceous filter aid).
The Celite mixture was purified by medium pressure silica gel chromatography (eluting with a gradient of 0 to 100% of ethyl acetate in hexanes) to provide the title compound as a white solid (17.8 g).
1H NMR (CDCl3): δ 8.10 (d, 2H), 7.46 (d, 2H), 3.76 (s, 2H).
19F NMR (CDCl3): δ -65.35.
References:
US2020/148672,2020,A1 Location in patent:Paragraph 0658-0660
5462-71-5
141 suppliers
$8.00/250mg

1418293-44-3
6 suppliers
inquiry
104-85-8
329 suppliers
$11.00/25g

1418293-44-3
6 suppliers
inquiry
19227-13-5
109 suppliers
$10.00/250mg

1418293-44-3
6 suppliers
inquiry
![Benzeneacetic acid, 4-[(hydroxyamino)iminomethyl]-](/CAS/20210305/GIF/199447-36-4.gif)