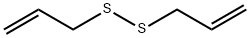
Diallyldisulfide synthesis
- Product Name:Diallyldisulfide
- CAS Number:2179-57-9
- Molecular formula:C6H10S2
- Molecular Weight:146.27
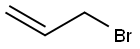
106-95-6
419 suppliers
$10.00/5g
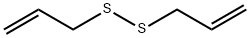
2179-57-9
391 suppliers
$6.00/5g
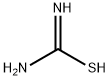
Yield:2179-57-9 95%
Reaction Conditions:
with 1,2-bis(5-methylisoxazol-3-yl)hydrazine;sodium carbonate;thiourea in water;acetonitrile at 80; for 2 h;
Steps:
Typical procedure for direct conversion of benzyl chloride to benzyl disulfides using thioureaand 4,4-azopyridine in CH3CN
General procedure: To a solution of thiourea (2.1 mmol, 0.160 g) and benzyl chloride (2 mmol, 0.23 mL) in wet CH3CN (3 mL CH3CN + 0.2 mL H2O), 4,4-azopyridine (1.1mmol, 0.202 g) and Na2CO3 (3 mmol, 0.318 g) were added. The mixture was stirred magneticallyat 80°C. The progress of the reaction was monitored by TLC or GC until the benzyl chloridewas consumed. After completion of the reaction, the mixture was filtered through a sintered glassfunnel to remove the produced pyridine hydrazine. The solvent was evaporated under reducedpressure and the so-obtained residue was purified by flash chromatography on silica gel withpetroleum ether as eluent to provide benzyl disulfide. Diallyl disulfide (4o) colorless oil; 1H NMR (250 MHz, CDCl3) δ 3.36 (d, J = 7.5 Hz, 2H),5.26 (m, 2H), 5.73 (m, 1H); 13C NMR (62.5 MHz, CDCl3): δ 133.1, 118.4, 42.7. Anal. Calcd.C6H10S2: C, 49.27%; H, 6.89%; S, 43.84%. Found: C, 49.18%; H, 7.05%; S, 43.77%.
References:
Khalili, Dariush;Iranpoor, Nasser;Firouzabadi, Habib [Journal of Sulfur Chemistry,2015,vol. 36,# 5,p. 544 - 555]
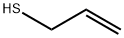
870-23-5
143 suppliers
$50.00/1 SAMPLE
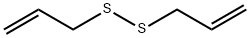
2179-57-9
391 suppliers
$6.00/5g
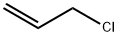
107-05-1
418 suppliers
$16.80/100ML
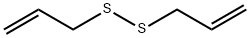
2179-57-9
391 suppliers
$6.00/5g