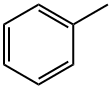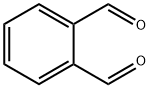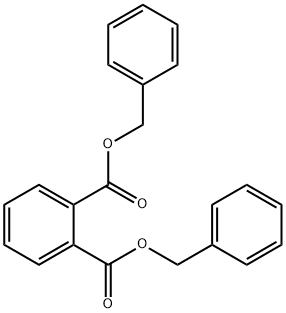
DIBENZYL PHTHALATE synthesis
- Product Name:DIBENZYL PHTHALATE
- CAS Number:523-31-9
- Molecular formula:C22H18O4
- Molecular Weight:346.38
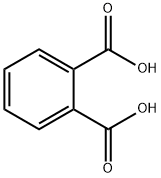
88-99-3
479 suppliers
$15.00/50 g

100-51-6
1432 suppliers
$5.00/100g

523-31-9
96 suppliers
$69.20/5g
Yield: 66%
Reaction Conditions:
with diphenyl hydrogen phosphate in toluene at 125; for 9 h;Inert atmosphere;
Steps:
3 Preparation of Dibenzyl Phthalate in Accordance with the Process of the Present Disclosure
In a flask 4.15 gm of phthalic acid, 5.947 gm (5.7 ml) of benzyl alcohol, and 624 gm of diphenylphosphoric acid were placed under nitrogen atmosphere.
50 ml toluene was added in the flask under nitrogen atmosphere to obtain a mixture.
The mixture was refluxed at 125° C. for 9 hours.
Water formed during the reaction was separated azeotropically by using Dean-stark apparatus.
The resultant mixture formed during the reaction was monitored by thin layer chromatography (TLC).
After completion of the reaction (monitored by TLC), the resultant mixture was cooled to 25° C. and quenched with water to obtain a product mixture.
To separate product from the product mixture, 100 ml of ethyl acetate was added to the product mixture to obtain a biphasic system.
A first organic layer, i.e. ethyl acetate layer was separated.
The aqueous layer was washed twice with ethyl acetate (50 ml each) followed by separation of second and third organic layer.
The separated organic layers were combined, concentrated, and dried over anhydrous sodium sulphate to obtain a crude product.
The crude product was purified using silica gel based flash chromatography using a solvent system containing 25% ethyl acetate in hexane to obtain dibenzyl phthalate having 96% purity.
The yield of dibenzyl phthalate was 66%.
References:
Reliance Industries Limited;Gupta, Virendrakumar;Phapale, Vilas Bhaskar US2018/86689, 2018, A1 Location in patent:Paragraph 0038-0040