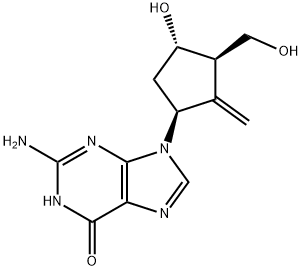
Entecavir synthesis
- Product Name:Entecavir
- CAS Number:142217-69-4
- Molecular formula:C12H15N5O3
- Molecular Weight:277.28

1383812-22-3
0 suppliers
inquiry

142217-69-4
487 suppliers
$39.00/5mg
Yield:142217-69-4 60%
Reaction Conditions:
Stage #1: ((1 R,3S,5S)-5-acetoxy-3-(2-amino-6-chloro-9H-purin-9-yl)-2-methylenecyclopentyl)methyl acetatewith sodium hydroxide at 80; for 2 h;
Stage #2: with hydrogenchloride in water at 0; pH=7;
Steps:
14
Example 14 Preparation of entecavir To (II) (0.4 g, 1.05 mmol) at room temperature, a 2.5 M solution of NaOH (10 mL) was added. The reaction mixture was heated to 80 °C for 2 h. The reaction mixture was cooled to 0 °C and neutralized with HCl to pH = 7. Decolorizing carbon (0.5 g) was added and the mixture was heated at 90 °C for 1 h. The hot mixture was filtered through Celite. The filtrate was cooled under stirring to effect crystallization. The estimated yield was 60 % approximately.
References:
EP2474548,2012,A1 Location in patent:Page/Page column 19
![6H-Purin-6-one, 9-[(1S,3R,4S)-4-(acetyloxy)-2-methylene-3-[[(4-nitrobenzoyl)oxy]methyl]cyclopentyl]-2-amino-1,9-dihydro-](/CAS/20210305/GIF/1435752-55-8.gif)
1435752-55-8
0 suppliers
inquiry

142217-69-4
487 suppliers
$39.00/5mg
1383811-86-6
0 suppliers
inquiry

142217-69-4
487 suppliers
$39.00/5mg
![D-erythro-Hept-1-ynitol, 6,7-anhydro-1,2,4-trideoxy-3-O-[(1,1-dimethylethyl)dimethylsilyl]-, (6ξ)-](/CAS/20211123/GIF/1383811-98-0.gif)
1383811-98-0
0 suppliers
inquiry

142217-69-4
487 suppliers
$39.00/5mg
![1-Hepten-6-yn-3-ol, 5-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-7-(trimethylsilyl)-, (3S,5R)-](/CAS/20210305/GIF/1383811-90-2.gif)
1383811-90-2
0 suppliers
inquiry

142217-69-4
487 suppliers
$39.00/5mg