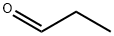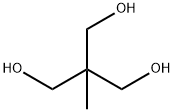
1,1,1-Tris(hydroxymethyl)ethane synthesis
- Product Name:1,1,1-Tris(hydroxymethyl)ethane
- CAS Number:77-85-0
- Molecular formula:C5H12O3
- Molecular Weight:120.15
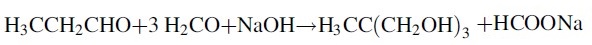
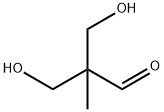
18516-18-2
7 suppliers
inquiry

77-85-0
256 suppliers
$6.00/25g
Yield:-
Reaction Conditions:
Stage #1: 2,2-dimethylolpropanalwith hydrogen in methanol at 60 - 110; under 15001.5 - 45004.5 Torr;
Stage #2: with hydrogen at 140 - 180; under 15001.5 - 45004.5 Torr;Temperature;
Steps:
1
1)In the impinging stream micro-mixing reactor by adding the total volume of 2/3 of the volume of water or recovery of dilute formaldehyde aqueous solution,According to the following ratio; formaldehyde, propionaldehyde,Triethylamine and soft water were added to the condensate kettle at a molar ratio of 2.5: 1: 0.6: 20,The concentration of the condensate is controlled at 30-40 ° C. The pH of the condensate may fluctuate due to side reactions,The pH of the condensation kettle is controlled at 8.5 to 8.9. The residence time is 8 to 12 hours,The resulting solution containing 8% -18% 2,2-dimethylolpropanal,1.5% -3% unreacted formaldehyde,0.5% to 1.5% of base,1% to 3% mono-diacetal and lipid compounds, 75% to 85% water;2) The unreacted excess formaldehyde and organic base and water are removed by distillation under reduced pressure from the condensate prepared in step 1)To obtain 50% to 70% of 2,2-dimethylol propionaldehyde, Evaporative condensate recovery can be used as a medium for the condensation process required for soft water,3) a concentrated solution prepared in step 2) is subjected to a first hydrogenation reaction in a first hydrogenation reactor to obtain a hydrogen-rich liquid,A hydrogenation reaction pressure of 2-6MPa, temperature 60-110 , the liquid space velocity of 0.4-0.8m3 / m3 · h,Gas airspeed 300-600Nm3 / m3 · h,The addition of 20% crude methanol to the concentrate favors the hydrogenation of 2,2-dimethylol propionaldehyde;4) The hydrogen-rich liquid obtained in step 3) is subjected to a second hydrogenation reaction in a second hydrogenation reactor,To obtain a hydrogenated mixed gas-liquid mixture; said step 4) a second hydrogenation reaction catalyst of 250-325 mesh copper-chromium-aluminum-zinc catalyst,The second hydrogenation reaction pressure is 2-6MPa, the temperature is 140-180 , the air velocity of the liquid is 0.1-0.25m3 / m3 · h;5) The gas-liquid mixture obtained in step 4) is separated into a separation column by gas-liquid separation and then refined in a rectifierTo give trimethylol ethane.The step (5) of the gas-liquid mixture is cooled and cooled to separate the high-pressure gas-liquid mixture, the gas is used for excess hydrogen,The liquid is decompressed into the pressurized distillation column, the column top pressure is 0.2MPa, the bottom temperature is 120-140 ,The top part of the top of the tower to control the top temperature 90-105 ; when the distillation bottom of the bottom of the sample analysis of the specific gravity of 100 above the specific gravity of 1.075 or more when the recovery into the crystallization tank crystallization, centrifugation, drying,The mother liquor is recycled to the distillation column. Finished product results are shown in Table 1,Dimethylol ethane finished gas chromatogram shown in Figure 1,Triethylamine as the condensation catalyst Step 2) The chromatogram of the concentrate is shown in Fig.
References:
CN106278817,2017,A Location in patent:Paragraph 0008; 0075-0078
![(2,2,5-TRIMETHYL-[1,3]DIOXAN-5-YL)METHANOL](/CAS/GIF/3663-46-5.gif)
3663-46-5
24 suppliers
$50.00/1g

77-85-0
256 suppliers
$6.00/25g
85963-60-6
0 suppliers
inquiry

77-85-0
256 suppliers
$6.00/25g