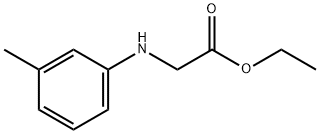
ethyl 2-[(3-methylphenyl)amino]acetate synthesis
- Product Name:ethyl 2-[(3-methylphenyl)amino]acetate
- CAS Number:21911-66-0
- Molecular formula:C11H15NO2
- Molecular Weight:193.24
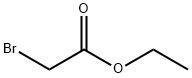
105-36-2
349 suppliers
$5.00/5 g
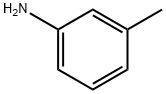
108-44-1
386 suppliers
$9.00/1g
![ethyl 2-[(3-methylphenyl)amino]acetate](/CAS/GIF/21911-66-0.gif)
21911-66-0
19 suppliers
$144.00/50mg
Yield: 94%
Reaction Conditions:
with potassium carbonate in tetrahydrofuran for 24 h;Reflux;
Steps:
ethyl m-tolylglycinate (7b)
To a THF (5 mL) solution of ethyl 2-bromoacetate (220 uL, 2 mmol), m- toluidine (214 uL, 2 mmol) and K2CO3 (830 mg, 6mmol) were added. After refluxing for 24 h, the reaction mixture was diluted with ethyl acetate and the organic phase was washed with water and brine, dried over MgSCb, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel (ethyl acetate / re-hexane = 1 :5) to afford ethyl /re-tolylglycinate (7b) (364mg, 94%). *H NMR (600 MHz, CDC13) d 7.11 (td, 1H, J = 7.8 and 1.2 Hz), 6.26(t, 1H, J = 7.8 Hz), 6.49 (s, 2H), 4.26 (q, 2H, / = 7.2 Hz), 3.91 (t, 2H, / = 1.8 Hz), 2.29 (s, 3H), 1.31 (t, 3H, / = 7.2 Hz); 13C NMR (150 MHz, CDCI3) d 171.1, 146.8, 139.2, 129.2, 119.5, 114.1, 110.4, 61.3, 46.1, 21.6, 14.2
References:
INDIANA UNIVERSITY RESEARCH AND TECHNOLOGY CORPORATION;GACHON UNIVERSITY OF INDUSTRY-ACADEMIC COOPERATION FOUNDATION;CORSON, Timothy W.;SEO, Seung-Yong;LEE, Bit WO2019/213076, 2019, A1 Location in patent:Page/Page column 57; 58
623-73-4
151 suppliers
$26.69/5g

108-44-1
386 suppliers
$9.00/1g
![ethyl 2-[(3-methylphenyl)amino]acetate](/CAS/GIF/21911-66-0.gif)
21911-66-0
19 suppliers
$144.00/50mg
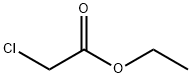
105-39-5
426 suppliers
$10.00/5g

108-44-1
386 suppliers
$9.00/1g
![ethyl 2-[(3-methylphenyl)amino]acetate](/CAS/GIF/21911-66-0.gif)
21911-66-0
19 suppliers
$144.00/50mg

60-29-7
0 suppliers
$30.00/50g

105-39-5
426 suppliers
$10.00/5g

108-44-1
386 suppliers
$9.00/1g
![ethyl 2-[(3-methylphenyl)amino]acetate](/CAS/GIF/21911-66-0.gif)
21911-66-0
19 suppliers
$144.00/50mg