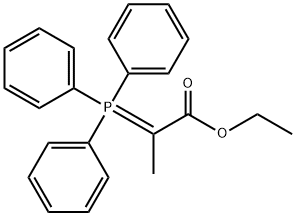
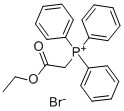
Ethyl 2-(triphenylphosphoranylidene)propionate synthesis
- Product Name:Ethyl 2-(triphenylphosphoranylidene)propionate
- CAS Number:5717-37-3
- Molecular formula:C23H23O2P
- Molecular Weight:362.4
Yield:5717-37-3 96%
Reaction Conditions:
in toluene at 80; for 12 h;Green chemistry;Solvent;Temperature;Reagent/catalyst;
Steps:
2 Preparation of (carbethoxyethylidene)triphenylphosphorane
To 1000mL three-necked flask was added 131g of triphenylphosphine and 100g 2- bromopropionate, was added 200mL of toluene and then 500ml water, 80 ° C the reaction was stirred 12h. After completion of the reaction was cooled to 50 ° C, the aqueous layer was separated, washed with 50ml of toluene, cooled to room temperature, a solution of 10% potassium hydroxide solution was adjusted ΡΗ = 8.0-8.5, stirring was continued for lh. Filtered and the resulting cake was dried at 50 ° C, to give (carbethoxyethylidene)triphenylphosphorane 175 g of solid, yield 96%
References:
CN103910759,2016,B Location in patent:Paragraph 0026; 0027; 0034
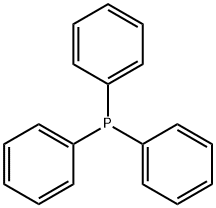
603-35-0
732 suppliers
$5.00/5G

5717-37-3
452 suppliers
$6.00/5g
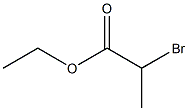
41978-69-2
2 suppliers
inquiry

5717-37-3
452 suppliers
$6.00/5g
1530-45-6
255 suppliers
$5.00/5g

5717-37-3
452 suppliers
$6.00/5g