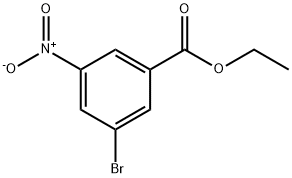
Ethyl 3-bromo-5-nitrobenzoate synthesis
- Product Name:Ethyl 3-bromo-5-nitrobenzoate
- CAS Number:690260-94-7
- Molecular formula:C9H8BrNO4
- Molecular Weight:274.07

64-17-5
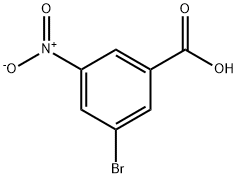
6307-83-1

690260-94-7
Add 3-bromo-5-nitrobenzoic acid (25 g, 101.6 mmol) and anhydrous ethanol (200 mL) to a 500 mL round bottom flask. With stirring at 0 °C, sulfoxide chloride (15 mL) was added slowly and dropwise. The reaction mixture was warmed to 80 °C with continuous stirring for 4 hours. Upon completion of the reaction, the reaction was quenched by the addition of 50 mL of water. The reaction mixture was extracted with dichloromethane (3 x 50 mL), the organic layers were combined and concentrated under reduced pressure. The concentrated residue was purified by silica gel column chromatography with ethyl acetate/petroleum ether as eluent (gradient elution, 1:20 to 1:10). Ethyl 3-bromo-5-nitrobenzoate 27.5 g (99% yield) was obtained as a white solid.

64-17-5
825 suppliers
$10.00/50g

6307-83-1
221 suppliers
$5.00/250mg

690260-94-7
70 suppliers
$17.00/250mg
Yield:690260-94-7 99%
Reaction Conditions:
with thionyl chloride at 0 - 80;
Steps:
1 Step 1: Ethyl 3-bromo-5-nitrobenzoate
Into a 500-mL round-bottom flask, was placed 3-bromo-5-nitrobenzoic acid (25 g, 101.6 mmol), EtOH (200 mL). This was followed by the addition of SOCl2 (15 mL) dropwise with stirring at 0oC. The resulting solution was stirred for 4 h at 80oC and then was quenched by the addition of 50 mL water. The resulting solution was extracted with 3x50 mL of DCM and the organic layers combined and concentrated under vacuum. The residue was applied onto a silica gel column and eluted with ethyl acetate/petroleum ether (1:20 to 1:10). This resulted in 27.5 g (99%) of the title compound as a white solid.
References:
WO2017/184623,2017,A1 Location in patent:Page/Page column 127

64-17-5
825 suppliers
$10.00/50g
51339-40-3
9 suppliers
inquiry

690260-94-7
70 suppliers
$17.00/250mg

6307-83-1
221 suppliers
$5.00/250mg

690260-94-7
70 suppliers
$17.00/250mg
121-92-6
601 suppliers
$6.00/25g

690260-94-7
70 suppliers
$17.00/250mg