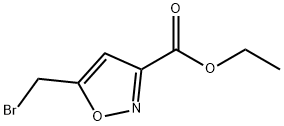
ETHYL 5-(BROMOMETHYL)ISOXAZOLE-3-CARBOXYLATE synthesis
- Product Name:ETHYL 5-(BROMOMETHYL)ISOXAZOLE-3-CARBOXYLATE
- CAS Number:84654-29-5
- Molecular formula:C7H8BrNO3
- Molecular Weight:234.05
Yield:84654-29-5 87%
Reaction Conditions:
with Sodium hydrogenocarbonate in water monomer;ethyl acetate at 20; for 24 h;
Steps:
6.2
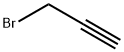
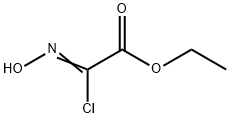
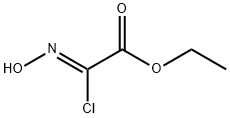
Method 2; A solution of ethyl 2-chloro-2-(hydroxyimino)acetate (1 1 g; 70.41 mmol) in ethyl acetate (60 mL) was added dropwise at room temperature to a mixture of 3-bromoprop-1-yne (15.2 mL; 141 mmol), sodium bicarbonate (11.95 g; 141 mmol), ethyl acetate (400 mL), and water (4 mL). The mixture was stirred at room temperature for 24 h and the solid was removed by filtration and washed with ethyl acetate. The filtrate was concentrated under reduced pressure and the residue was purified by flash chromatography on silica gel (eluent: 15 to 100% of dichloromethane in heptane) to give 14.38 g (87%) of ethyl 5- (bromomethyl)isoxazole-3-carboxylate as a white solid. ESI/APCI(+): 234 (M+H).1H NMR (CDCI3) 6.74 (s, 1 H); 4.50 (s, 2H); 4.45 (q, 2H); 1.42 (t, 3H).
References:
WO2010/142801,2010,A1 Location in patent:Page/Page column 148
123770-62-7
72 suppliers
$39.00/100mg

84654-29-5
28 suppliers
inquiry
3209-72-1
187 suppliers
$11.00/250mg

84654-29-5
28 suppliers
inquiry