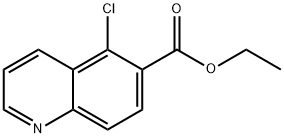
Ethyl 5-chloroquinoline-6-carboxylate synthesis
- Product Name:Ethyl 5-chloroquinoline-6-carboxylate
- CAS Number:180421-62-9
- Molecular formula:C12H10ClNO2
- Molecular Weight:235.67

64-17-5
815 suppliers
$10.00/50g
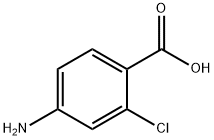
2457-76-3
300 suppliers
$5.00/1g

56-81-5
1754 suppliers
$5.00/25g

180421-62-9
6 suppliers
inquiry
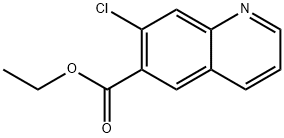
916812-04-9
5 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-amino-2-chlorobenzoic acid;glycerolwith sulfuric acid;sodium 3-nitrobenzenesulfonate in water at 100 - 140; for 4 h;
Stage #2: ethanol in water at 60;
Stage #3: with ammonia in water;
Steps:
8.a
Ethyl 7-chloro-6-quinolinecarboxylate and ethyl 5-chloro-6-quinolinecarboxylate.A solution of 4-amino-2-chlorobenzoic acid (17.1 g; 0.1 mol.), glycerol (41.4 g; 0.45 mol.), sodium 3-nitrobenzenesulfonate (45.0 g; 0.2 mol.) and 75% aq. sulfuric acid (250 mL) was stirred and heated at 100 0C for 3 h then at 1400C for 1 h. The mixture was allowed to cool to 60 ° then ethanol was added and the EPO
References:
WO2006/132739,2006,A2 Location in patent:Page/Page column 37-38