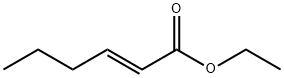
Ethyl (E)-hex-2-enoate synthesis
- Product Name:Ethyl (E)-hex-2-enoate
- CAS Number:27829-72-7
- Molecular formula:C8H14O2
- Molecular Weight:142.2

123-72-8
438 suppliers
$12.32/25ML
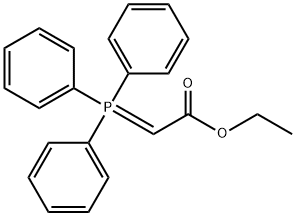
1099-45-2
371 suppliers
$6.00/5g

27829-72-7
196 suppliers
$16.00/10g
Yield:27829-72-7 72%
Reaction Conditions:
in dichloromethane at 20; for 47 h;Inert atmosphere;Wittig Olefination;
Steps:
Synthesis and data for (E)-ethyl hex-2-enoate (6):
To a stirred solution of butanal(0.213 g, 2.95 mmol) in CH2Cl2 (15 mL) under an atmosphere of nitrogen wasadded (carbethoxymethylene)triphenylphosphorane (1.24 g, 3.50 mmol) andthe resulting mixture stirred for 47 h. The solvent was removed in vacuo,without application of heat, and pentane (30 mL) was added to the residue,which was then stirred for 30 min. The resulting mixture was filtered, thesolvent removed in vacuo, without application of heat, and the crude productwas purified using flash chromatography (95:5 pentane/Et2O) to give the ester6 (0.30 g, 72%) as a pale yellow oil. dH (400 MHz, CDCl3): 0.93 (3H, t, J = 7.5 Hz,6-CH3), 1.28 (3H, t, J = 7.0 Hz, 8-CH3), 1.44-1.53 (2H, m, 5-CH2), 2.14-2.20 (2H,m, 4-CH2), 4.18 (2H, q, J = 7.0 Hz, 7-CH2), 5.81 (1H, dt, J = 1.5, 15.9 Hz, 2-H), 6.96(1H, dt, J = 7.1, 15.2 Hz, 3-H); dC (100 MHz, CDCl3): 13.8 (C-6), 14.4 (C-8), 21.4(C-5), 34.3 (C-4), 60.2 (C-7), 121.5 (C-2), 149.3 (C-3), 166.9 (C-1).
References:
Duhamel, Nina;Piano, Federico;Davidson, Samuel J.;Larcher, Roberto;Fedrizzi, Bruno;Barker, David [Tetrahedron Letters,2015,vol. 56,# 13,p. 1728 - 1731]

6728-26-3
359 suppliers
$19.00/25g

64-17-5
823 suppliers
$10.00/50g

27829-72-7
196 suppliers
$16.00/10g
867-13-0
484 suppliers
$10.00/1g

123-72-8
438 suppliers
$12.32/25ML

27829-72-7
196 suppliers
$16.00/10g
2396-84-1
166 suppliers
$11.00/10g
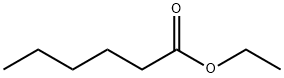
123-66-0
356 suppliers
$10.00/5g

27829-72-7
196 suppliers
$16.00/10g
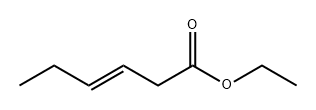
26553-46-8
24 suppliers
inquiry
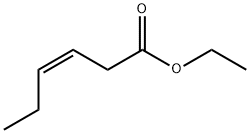
64187-83-3
5 suppliers
inquiry

123-72-8
438 suppliers
$12.32/25ML

27829-72-7
196 suppliers
$16.00/10g
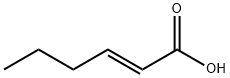
13419-69-7
272 suppliers
$10.00/1g