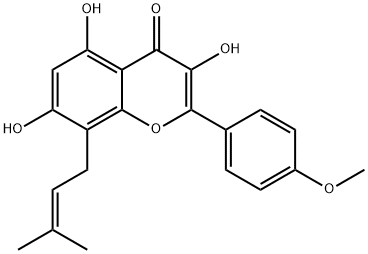
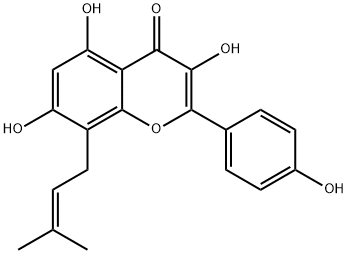
Icaritin synthesis
- Product Name:Icaritin
- CAS Number:118525-40-9
- Molecular formula:C21H20O6
- Molecular Weight:368.38
Total Synthesis of Icaritin via Microwave-assistance Claisen Rearrangement
113558-15-9
232 suppliers
$6.00/1mg

118525-40-9
229 suppliers
$5.00/5mg
Yield:118525-40-9 82%
Reaction Conditions:
with sulfuric acid in ethanol;water at 50; for 5 h;
Steps:
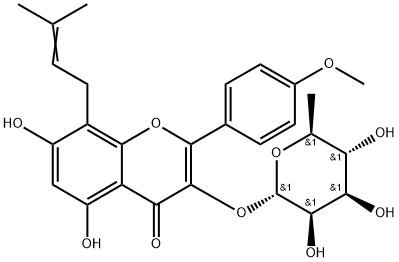
1.2 3,5,7-trihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-4H-chromen-4-one
Concentrated sulfuric acid (20 mL) was slowly added dropwise to a mixed solvent of ethanol and water (V / V = 1/1, 200 mL), followed by addition of 5,7-dihydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-3-(((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-4H-chromen-4-one (6 g, 11.7 mmol) and the reaction mixture was stirred at 50 ° C for 5 hours and concentrated under reduced pressure. The residue was added to 100 mL of water and stirred for 30 minutes. The mixture was extracted with ethyl acetate (50 mL x 3) and the combined organic layers were washed with saturated sodium chloride solution (30 mL) and dried over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure and the residue was recrystallized from ethyl acetate to give the title compound as a yellow solid (3.5 g, 82%).
References:
Guangdong East Sunshine Pharmaceutical Co., Ltd.;Zhang, Yingjun;Zhou, Pingjian;Wang, Xiaojun;Yang, Chuanwen CN104530127, 2016, B Location in patent:Paragraph 0156-0157; 0161-0162
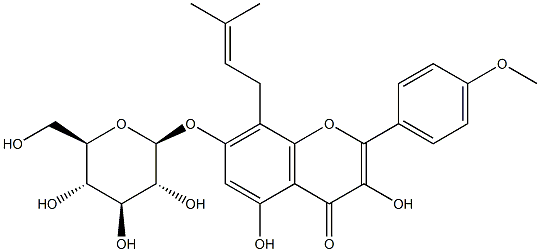
56725-99-6
143 suppliers
$48.00/5mg

118525-40-9
229 suppliers
$5.00/5mg
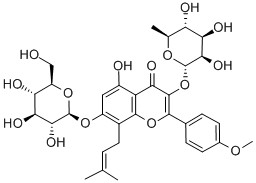
489-32-7
617 suppliers
$7.00/25mg

118525-40-9
229 suppliers
$5.00/5mg
28610-31-3
64 suppliers
$125.00/1 mg

77-78-1
311 suppliers
$19.69/1ml

118525-40-9
229 suppliers
$5.00/5mg
16274-11-6
44 suppliers
inquiry

118525-40-9
229 suppliers
$5.00/5mg