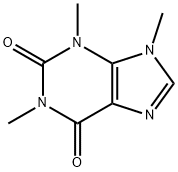
ISOCAFFEINE synthesis
- Product Name:ISOCAFFEINE
- CAS Number:519-32-4
- Molecular formula:C8H10N4O2
- Molecular Weight:194.19

64-18-6
1132 suppliers
$22.12/250ML
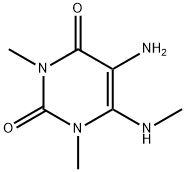
61541-46-6
1 suppliers
inquiry

519-32-4
73 suppliers
$372.00/25mg
Yield: 0.5 g
Reaction Conditions:
for 3 h;Reflux;Inert atmosphere;
Steps:
11.e e) l,3,9-Trimethyl-lH-purine-2,6(3H,9H)-dione (10)
A mixture of 5-amino-l,3-dimethyl-6-methylamino-pyrimidine-2,4(lH,3H)-dione (9) (1.5 g) and formic acid (10 mL) was refluxed for 3 h under nitrogen atmosphere. An excess of formic acid was evaporated under reduce pressure. The residue was extracted with CH2C12, washed with aq. Na2C03, dried over Na2S04 and evaporated to dryness. The residue was purified by column chromatography (eluent CH2C12 : MeOH, 10: 1) to give the title compound (0.5 g) as a white solid. NMR (400 MHz, DMSO~d6) δ (ppm): 3.22, 3.68 and 3.93 (all s, all 3H), 7.66 (s, 1 H). MS (EI) mlz: 195 |M+2]+
References:
LATVIAN INSTITUTE OF ORGANIC SYNTHESIS;ARSENJANS, Pavels;VASILJEVA, Jelena;DOMRACHEVA, llona;SHESTAKOVA, Irina;GULBE, Anita;KANEPE-LAPSA, Iveta;KAUSS, Valerjans;KALVINS, Ivars WO2016/159747, 2016, A1 Location in patent:Page/Page column 13; 14
2564-54-7
2 suppliers
inquiry

519-32-4
73 suppliers
$372.00/25mg
5440-00-6
127 suppliers
$65.00/1 g

519-32-4
73 suppliers
$372.00/25mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

519-32-4
73 suppliers
$372.00/25mg
58-55-9
621 suppliers
$5.00/1g

519-32-4
73 suppliers
$372.00/25mg