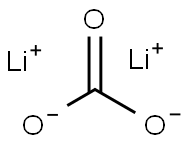
Lithium carbonate synthesis
- Product Name:Lithium carbonate
- CAS Number:554-13-2
- Molecular formula:CLi2O3
- Molecular Weight:73.89
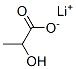
867-55-0
186 suppliers
$42.86/100g

554-13-2
672 suppliers
$5.00/100mg
Yield:-
Reaction Conditions:
with ammonium carbonate;sodium hydroxide in ethanol;water at 20 - 50;Reagent/catalyst;
Steps:
7 Production of lithium carbonate and complex from lithium lactate
General procedure: 50 mL of an aqueous solution of sodium lactate (2 M, 100 mmoles) and sodiumhydroxide (2 M, 100 mmoles) was added to ethanol (50 mL) and stirred at room temperature. Ammonium carbonate (10.57 g, 110 mmoles) was added resulting in a fine, white precipitate. The precipitate was filtered off under vacuum and dried in an oven at 50 °C overnight to yield a mass of dry solid equivalent to a 95.5% recovery of sodium carbonate. Analysis of the solid indicated the presence of 3 % of lactate (assumed to besodium lactate).To the lithium lactate filtrate obtained in Example 4, experiment 2, above, was added ammonium carbonate (3 molar equivalents). A suspension formed, the mixturewas stirred for 40 minutes at ambient temperature and filtered. The solid obtained was washed with water (50 mL) and dried at 50 °C for 18 hours to afford 1 .98g of solid.
References:
WO2015/155535,2015,A1 Location in patent:Page/Page column 18

124-38-9
134 suppliers
$214.00/14L

554-13-2
672 suppliers
$5.00/100mg
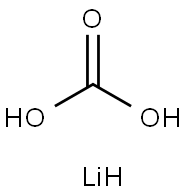
5006-97-3
3 suppliers
inquiry

554-13-2
672 suppliers
$5.00/100mg
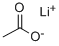
546-89-4
323 suppliers
$6.00/25g

554-13-2
672 suppliers
$5.00/100mg
553-91-3
195 suppliers
$14.00/1g

554-13-2
672 suppliers
$5.00/100mg