Potassium bicarbonate synthesis
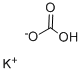
- Product Name:Potassium bicarbonate
- CAS Number:298-14-6
- Molecular formula:CHKO3
- Molecular Weight:100.12
Potassium bicarbonate also occurs naturally in the mineral calcinite.

124-38-9
134 suppliers
$214.00/14L
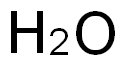
7732-18-5
507 suppliers
$13.50/100ML
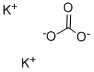
584-08-7
1252 suppliers
$5.00/100g

298-14-6
504 suppliers
$6.00/100g
Yield:-
Reaction Conditions:
in ethylene glycol;diethylene glycol at 22; for 1 h;
Steps:
5 Example V
This Example V demonstrates soluble catalyst recycle and the use of carbon dioxide to reformulate bicarbonate catalyst that is degraded to carbonate form during the distillation recovery of alkylene oxide product. A 500-ml stirred batch reactor was charged with a mixture of 15 wt% MEG in water containing 7.1 wt% potassium bicarbonate soluble catalyst. The mixture was heated under N2 atmosphere (7000 kPa) to 80°C, before the addition of 18 grams of ethylene oxide. Conversion was 99.1% after 4 hours, corresponding to a rate constant (adjusted to 90°C) of 2.7 1/h/(eq/L) for the catalyzed reaction, relative to the thermal reaction. The mixture was distilled in a batch still to remove water and a portion of the MEG product, with a maximum bottoms temperature of 136°C, over a period of 6.5 hours. No precipitates were observed in the bottoms produced while heated at distillation temperature. A final bottoms fraction containing MEG and trade DEG (diethylene glycol) yielded 36.6 grams. Titration of the bottoms fraction indicated 91% retention of potassium carbonate or bicarbonate catalyst. Small loss of catalyst can be attributed to sampling for analysis. 93% of the bicarbonate had degraded to carbonate via loss of carbon dioxide during distillation. The bottoms mixture containing degraded catalyst was blended with an amount of water required for a second reaction cycle, and sparged with carbon dioxide for one hour at ambient temperature (22°C). Titration revealed virtually complete regeneration of bicarbonate, with 97.5% in bicarbonate form vs. 2.5% carbonate. 244 grams of the water-diluted recycle mixture were recycled to the reactor for a second reaction, conducted at 90°C, and also entailing addition of 18 grams of ethylene oxide. A conversion of 99.3% was obtained in only 3 hours, for a calculated catalytic rate constant of 3.0 1/h/(eq/L). Titration of the reaction mixture indicated 96.0% of the catalyst remaining in bicarbonate form, indicating negligible catalyst degradation during reaction. Reaction selectivity to MEG was 96%, indicating successful recycle and regeneration of selective bicarbonate catalyst. Rate constants for the recycle demonstration experiment compare favorably with the smaller-scale results reported in Table 1 of above Example I.
References:
EP1484300,2004,A1 Location in patent:Page 11; 12

124-38-9
134 suppliers
$214.00/14L

298-14-6
504 suppliers
$6.00/100g

124-38-9
134 suppliers
$214.00/14L

584-08-7
1252 suppliers
$5.00/100g

298-14-6
504 suppliers
$6.00/100g
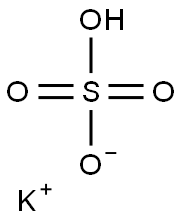
7646-93-7
246 suppliers
$10.00/10g

298-14-6
504 suppliers
$6.00/100g