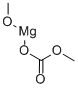
MAGNESIUM METHYL CARBONATE synthesis
- Product Name:MAGNESIUM METHYL CARBONATE
- CAS Number:4861-79-4
- Molecular formula:C3H6MgO4
- Molecular Weight:130.38

67-56-1
790 suppliers
$7.29/5ml-f

124-38-9
134 suppliers
$175.00/23402
7439-95-4
0 suppliers
$11.60/0.9g

4861-79-4
37 suppliers
$95.00/100mL
Yield:-
Reaction Conditions:
Stage #1:methanol;magnesiumInert atmosphere;
Stage #2:carbon dioxide in N,N-dimethyl-formamide
Steps:
3 Example 3: Synthesis of Methylmagnesium Carbonate (MMC)
Methylmagnesium carbonate (MMC) was synthesized following the protocol disclosed by Balasubrahmanyam et al., (1973), Organic Synthesis, Collective Volume V, John Wiley & Sons, Inc., p. 439-444. (0135) A dry 2 L, three necked flask was fitted with a mechanical stirrer, a condenser, and a IL, pressure-equalizing addition funnel, the top of which was fitted with a gas inlet tube. A clean, dry magnesium ribbon (40.0 g, 1.65 mol) was placed in the flask and the system was flushed with nitrogen prior to the addition of anhydrous methanol (600 mL). The evolution of hydrogen gas was controlled by cooling the reaction mixture. When hydrogen evolution had ceased, a slow stream of nitrogen was passed through the system and the condenser was replaced by a total condensation-partial take-off distillation head. The nitrogen flow was stopped and the bulk of the methanol distilled from the solution under reduced pressure. Distillation was stopped when stirring of the pasty suspension of magnesium methoxide was no longer practical. The system was again flushed using nitrogen and the outlet from the distillation head was attached to a small trap containing mineral oil so that the volume of gas escaping from the reaction system could be estimated. (0136) Anhydrous dimethylformamide (DMF)(700 mL) was added to the reaction flask, and the resulting suspension was stirred vigorously while a stream of anhydrous carbon dioxide was passed into the reaction vessel through the gas inlet tube attached to the addition funnel. The dissolution of carbon dioxide was accompanied by an exothermic reaction with the suspended magnesium methoxide. When no more CO2 is absorbed, the colorless solution was heated under a slow stream of CO2 gas until the temperature of the liquid distilling reached 140° C., indicating that residual methanol had been removed from the reaction mixture. The reaction mixture was flushed using a slow stream of nitrogen to aid in cooling the mixture to room temperature under an inert atmosphere. This yielded a solution having 536 mg MMC/mL of DMF.
References:
Full Spectrum Laboratories Ltd;Kavarana, Malcolm J.;Peet, Richard C. US2017/283837, 2017, A1 Location in patent:Paragraph 0134; 0135; 0136

67-56-1
790 suppliers
$7.29/5ml-f

124-38-9
134 suppliers
$175.00/23402

4861-79-4
37 suppliers
$95.00/100mL