n-Butane
- CAS No.
- 106-97-8
- Chemical Name:
- n-Butane
- Synonyms
- BUTANE;r600;n-Butan;Batane;n-C4H10;BUTANES;1-Butane;A-17;Q GAS;Bu-Gas
- CBNumber:
- CB6152626
- Molecular Formula:
- C4H10
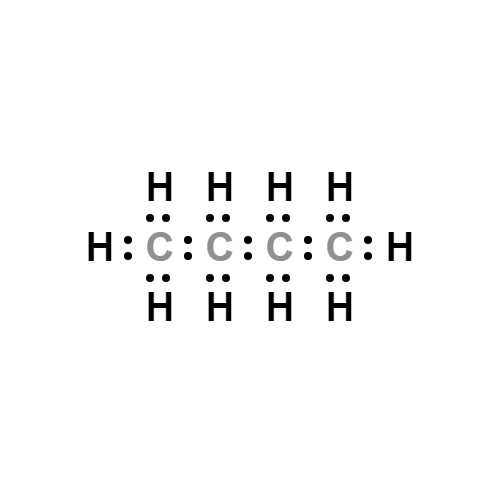
Lewis structure
- Molecular Weight:
- 58.12
- MDL Number:
- MFCD00009424
- MOL File:
- 106-97-8.mol
- MSDS File:
- SDS
| Melting point | −138 °C(lit.) |
|---|---|
| Boiling point | −0.5 °C(lit.) |
| Density | 0.579 g/mL at 20 °C(lit.) |
| vapor density | 2.11 (vs air) |
| vapor pressure | 3.21, 1.26, and 0.66 mM at 4, 25, and 50 °C, respectively (Kresheck et al., 1965) |
| refractive index | 1.3326 |
| Flash point | 45 |
| form | gas |
| Odor | faint disagreeable odor |
| Odor Threshold | 1200ppm |
| Water Solubility | 73.24mg/L(25 ºC) |
| Merck | 1515 |
| BRN | 969129 |
| Henry's Law Constant | (atm?m3/mol): 0.356 at 5 °C, 0.454 at 10 °C, 0.568 at 15 °C, 0.695 at 20 °C, 0.835 at 25 °C (Ben-Naim et al., 1973) |
| Exposure limits | TLV-TWA 800 ppm (~1920 mg/m3) (ACGIH), 500 ppm (1200 mg/m3) (MSHA). |
| Dielectric constant | 1.4(-1℃) |
| Stability | Stable. Extremely flammable. Readily forms explosive mixtures with air. Note low flash point. Incompatible with strong oxidizing agents, strong acids, strong alkalies. |
| LogP | 2.890 |
| CAS DataBase Reference | 106-97-8(CAS DataBase Reference) |
| FDA 21 CFR | 582.1165 |
| Substances Added to Food (formerly EAFUS) | N-BUTANE |
| EWG's Food Scores | 4-7 |
| NCI Dictionary of Cancer Terms | butane |
| FDA UNII | 6LV4FOR43R |
| EPA Substance Registry System | Butane (106-97-8) |
| Cosmetics Info | Butane |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS02,GHS04 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H220-H280 | |||||||||
| Precautionary statements | P410+P403 | |||||||||
| Hazard Codes | F+,F,T | |||||||||
| Risk Statements | 12-46-45 | |||||||||
| Safety Statements | 9-16-45-53 | |||||||||
| RIDADR | UN 2037 2.1 | |||||||||
| WGK Germany | - | |||||||||
| RTECS | EJ4200000 | |||||||||
| F | 4.5-31 | |||||||||
| Hazard Note | Extremely Flammable | |||||||||
| DOT Classification | 2.1 (Flammable gas) | |||||||||
| HazardClass | 2.1 | |||||||||
| HS Code | 2901100000 | |||||||||
| Toxicity | LC50 (inhalation) for mice 680 gm/m3/2-h, rats 658 gm/m3/4-h (quoted, RTECS, 1985). | |||||||||
| IDLA | 1,600 ppm (>10% LEL) | |||||||||
| NFPA 704 |
|
n-Butane price More Price(6)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 494402 | Butane 99% | 106-97-8 | 170g | $376 | 2024-03-01 | Buy |
| Cayman Chemical | 25932 | Butane Residual Solvent Standard | 106-97-8 | 1mL | $40 | 2023-01-06 | Buy |
| SynQuest Laboratories | 1100-1-X0 | Butane 98.0% | 106-97-8 | 25g | $45 | 2021-12-16 | Buy |
| SynQuest Laboratories | 1100-1-X0 | Butane 98.0% | 106-97-8 | 100g | $80 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0028739 | BUTANE 95.00% | 106-97-8 | 250ML | $4544.93 | 2021-12-16 | Buy |
n-Butane Chemical Properties,Uses,Production
Overview
N-Butane [C4H10] is a colorless gas with a faint petroleum-like odor. The main sources of butane are the refinery of crude oil and the processing of natural gas. It is commonly blended into motor vehicle gasoline to increase the fuel’s volatility and to make engine starting easier. Butane contains mixtures of methane, ethane, propane, isobutane, and n-butane and is a colourless aliphatic hydrocarbon gas with a gasoline-like odour. Butane is a component of liquefied petroleum gas (LPG) and as such is used in a wide variety of fuel applications for both recreational and leisure use, including heating and air conditioning, refrigeration, cooking, and lighters. Butane is commonly used alone or in mixtures as a propellant in aerosol consumer products, such as hairsprays, deodorants and antiperspirants, shaving creams, edible oil and dairy products, cleaners, pesticides, and coatings (e.g. automobile or household spray paint). Butane is used as a chemical intermediate in the production of maleic anhydride, ethylene, methyl tert-butyl ether (MTBE), synthetic rubber, and acetic acid and its by-products. Butane is a simple asphyxiant with explosive and flammable potential. It is also a widely used substance of abuse. The main target organs are in the CNS and cardiovascular system. Improper use and handling cause poisoning. Exposure to high levels of butane vapors can result in asphyxia. The symptoms of butane poisoning include but not limited to, rapid breathing and pulse rate, headache, dizziness, visual disturbances, mental confusion, incoordination, mood changes, muscular weakness, tremors, cyanosis, narcosis and numbness of the extremities, and unconsciousness leading to central nervous system injury.
Figure 1 Chemical structure of n-butane.
Production
Butane is extremely abundant in many parts of the world, being relatively inexpensive to produce and mine. It is a fossil fuel, which has been created over the course of millions of years by a complex process deep inside the earth from the remains of plants, animals, and numerous microorganisms[4]. Different types of machinery that require butane to operate seemed quite magical when they were developed long ago, but there really is little magic involved in butane production. Its production demands human ingenuity, hard work, repeatable production processes, and following safety procedures every step of the way[4]. General its production includes the following steps: removal of oil and condensate; remove the water; glycol dehydration.
Applications
n-Butane can be used in the production of ethylene and 1,3-butadiene. It can also be used as a chemical feedstock for special chemicals in the solvent, rubber, and plastics industries, in the blending of gasoline or motor fuel, as a constituent in liquefied petroleum gas [LPG], and as an extraction solvent in deasphalting processes[5, 6].
N-Butane can be used for the manufacture of ethylene and butadiene, a key ingredient of synthetic rubber[7]. N-butane [R600] is a kind of ozone depletion neutral refrigerant, being a potential refrigerant for household appliances. N-butane has a slightly higher Ranking COP level compared to isobutane and a much higher COP than R134a of which the latter is still used in household appliances around the world[8].
Warning and Risk
Inhaling of butane can cause various central nerve system effects including drowsiness, narcosis, asphyxia, headache, cardiac arrhythmia and frostbite, which can result in instant death from Asphyxiation, Acute toxicity and ventricular fibrillation. Skin and eyes contact may cause burn or frostbite[9, 10]. Butane is the most commonly misused volatile solvent in the UK, and was the cause of 52% of solvent related deaths in 2000[9].
References
- http://www.thermopedia.com/content/607/
- https://pubchem.ncbi.nlm.nih.gov/compound/butane#section=Top
- https://www.tceq.texas.gov/assets/public/implementation/tox/dsd/final/butanes.pdf
- https://butanesource.com/blog/79-how-butane-is-made
- https://www.tceq.texas.gov/assets/public/implementation/tox/dsd/final/butanes.pdf
- https://www.boconline.co.uk/en/products-and-supply/speciality-gas/pure-gases/n-butane/n-butane.html
- https://w3.siemens.com/mcms/sensor-systems/CaseStudies/CS_Butyl_Rubber_2013-01_en_Web.pdf
- https://docs.lib.purdue.edu/cgi/viewcontent.cgi?article=2791&context=icec
- http://bennettgroup.ca/SDS/data/Gas%20Products/Butane%20-%20w221.pdf
- https://www.worldofmolecules.com/fuels/butane.htm
Description
Butane is a flammable, colorless gas that follows propane in the alkane series. Butane is also called n-butane, with the “n” designating it as normal butane, the straight chain isomer. Butane’s other isomer is isobutane. The chemical name of isobutane is 2-methylpropane. Isomers are different compounds that have the same molecular formula. Normal butane and isobutane are two different compounds, and the name butane is used collectively to denote both n-butane and isobutane; the names n-butane and isobutane are used to distinguish properties and chemical characteristics unique to each compound. Butane, along with propane, is a major component of liquefied petroleum gas . It exists as a liquid under moderate pressure or below 0℃ at atmospheric pressure, which makes it ideal for storage and transportation in liquid form.
Description
Butane Residual Solvent Standard (Item No. 25932) is a certified reference material standard for butane, a solvent that has been used in the extraction of cannabinoids from Cannabis and has been identified as a contaminant in butane hash oil and Δ9-THC concentrates. It is designed for use as a reference standard for butane by GC- or LC-MS. This product is intended for research and forensic applications.
Chemical Properties
The main sources of butane are crude oil refi ning and natural gas processing. It is usually blended into motor vehicle gasoline to increase the fuel’s volatility and to make engine starting easier. Butane contains mixtures of methane, ethane, propane, iso-butane, and n-butane and is a colorless aliphatic hydrocarbon gas with a gasoline-like odor. Butane is a component of liquefi ed petroleum gas (LPG) and as such is used in a wide variety of fuel applications for both recreational and leisure use, including heating and air-conditioning, refrigeration, cooking, and in lighters. Butane is commonly used alone or in mixtures as a propellant in aerosol consumer products, such as hair sprays, deodorants and antiperspirants, shaving creams, edible oil and dairy products, cleaners, pesticides and coatings (e.g., automobile or household spray paint). Butane is used as a chemical intermediate in the production of maleic anhydride, ethylene, methyl tert-butyl ether (MTBE), synthetic rubber, and acetic acid and its by-products. Butane is a simple asphyxiant with explosive and flammable potential. It is also widely used as a substance of abuse. The main target organs are in the central nervous and cardio vascular systems. Butane is found in aerosols, lighter fuel and refi lls, small blow torches, and camping stoves. Pure grades of butane are used in calibrating instruments and as a food additive. It is widely available. Misuse and adulteration of butane is a common com mercial practice.
Physical properties
Colorless, flammable gas with a faint, disagreeable, natural gas or gasoline-like odor. Odor threshold concentration in air is 1,200 ppmv (Nagata and Takeuchi, 1990). Detected in water at a concentration of 6.2 mg/L (Bingham et al., 2001).
History
Butane is extracted from natural gas and is also obtained during petroleum refining. Butane can be obtained from natural gas by compression, adsorption, or absorption. All three processes were used in the early days of the LPG industry, but compression and adsorption were generally phased out during the 20th century. Most butane now is obtained from absorption and separation from oil.
Uses
Butane is the common fuel used in cigarette lighters and also as an aerosol propellant, a calibration gas, a refrigerant, a fuel additive, and a chemical feedstock in the petrochemical industry.
Uses
n-Butane occurs in petroleum, natural gas,and in refinery cracking products. It isused as a liquid fuel, often called liquefiedpetroleum gas, in a mixture with propane. Itis also used as a propellant for aerosols, a rawmaterial for motor fuels, in the production ofsynthetic rubber, and in organic synthesis.
Uses
n-Butane can be obtained from natural gas and from refinery hydro cracker streams. Most of the n-butane goes into fuel additive uses. The major chemical use is as a feedstock for ethylene production by cracking . The other important chemical uses for butane are in oxidation to acetic acid and in the production of maleic anhydride. In the past, butane also was the main feedstock for the production of butadiene by dehydrogenation, but it has been replaced by coproduct butadiene obtained from ethylene production.
Ethylene. The largest potential chemical market for n-butane is in steam cracking to ethylene and coproducts. n-Butane is a supplemental feedstock for olefin plants and has accounted for 1-4 percent of total ethylene production for most years since 1970. It can be used at up to 10-15 percent ofthe total feed in ethane/propane crackers with no major modifications . n-Butane can also be used as a supplemental feed at as high as 20-30 percent in heavy naphtha crackers. The consumption of C4S has fluctuated considerably from year to year since 1970, depending on the relative price ofbutane and other feedstocks. The yield of ethylene is only 36-40 percent, with the other products including methane, propylene, ethane, and butadiene, acetylene, and butylenes. About 2-3 billion Ib of butane are consumed annually to produce ethylene.
Uses
As producer gas; raw material for motor fuels, in the manufacture of synthetic rubbers.
Definition
A gaseous hydrocarbon,C4H10; d. 0.58 g cm–3; m.p. –138°C;b.p. 0°C. Butane is obtained frompetroleum (from refinery gas orby cracking higher hydrocarbons).The fourth member of the alkaneseries, it has a straight chain ofcarbon atoms and is isomeric with2-methylpropane (CH3CH(CH3)CH3,formerly called isobutane). It can easilybe liquefied under pressure and issupplied in cylinders for use as a fuelgas. It is also a raw material for makingbuta-1,3-diene (for synthetic rubber).
General Description
N-BUTANE is a colorless gas with a faint petroleum-like odor. For transportation N-BUTANE may be stenched. N-BUTANE is shipped as a liquefied gas under its vapor pressure. Contact with the liquid can cause frostbite. N-BUTANE is easily ignited. Its vapors are heavier than air. Any leak can be either liquid or vapor. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. N-BUTANE is used as a fuel, an aerosol propellant, in cigarette lighters, and to make other chemicals.
Air & Water Reactions
Highly flammable.
Reactivity Profile
N-BUTANE can explode when exposed to flame or when mixed with (nickel carbonyl + oxygen). N-BUTANE can also react with oxidizers. Strong acids and alkalis should be avoided. .
Hazard
Highly flammable, dangerous fire and explosion risk. Explosive limits in air 1.9–8.5%. Narcotic in high concentration. Central nervous sys- tem impairment.
Health Hazard
n-Butane is a nontoxic gas. Exposure toits atmosphere can result in asphyxia. Athigh concentrations it produces narcosis.Exposure to 1% concentration in air for10 minutes may cause drowsiness. Its odoris detectable at a concentration of 5000 ppm.
Health Hazard
Exposures to butane cause excitation, blurred vision, slurred speech, nausea, vomiting, coughing, sneezing, and increased salivation. With increased periods of exposure to high concentrations of butane, the signs and symptoms of toxicity become more severe. For instance, the exposed worker demonstrates confusion, perceptual distortion, hallucina tions (ecstatic or terrifying), delusions, behavioral changes, tinnitus, and ataxia. Workers exposed to larger doses of butane suffer from nystagmus, dysarthria, tachycardia, central depression of the CNS, drowsiness, coma, and sudden death. It has been reported that poisoned individuals show anoxia, vagal inhibition of the heart, respiratory depression, cardiac arrhythmias, and trauma.
Fire Hazard
EXTREMELY FLAMMABLE. Will be easily ignited by heat, sparks or flames. Will form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. CAUTION: Hydrogen (UN1049), Deuterium (UN1957), Hydrogen, refrigerated liquid (UN1966) and Methane (UN1971) are lighter than air and will rise. Hydrogen and Deuterium fires are difficult to detect since they burn with an invisible flame. Use an alternate method of detection (thermal camera, broom handle, etc.) Vapors may travel to source of ignition and flash back. Cylinders exposed to fire may vent and release flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket.
Flammability and Explosibility
Extremely flammable
Safety Profile
Mildly toxic by inhalation. Causes drowsiness. An asphyxlant. Very dangerous fire hazard when exposed to heat, flame, or oxidizers. Highly explosive when exposed to flame, or when mixed with [Ni(CO)4 + O2]. To fight fire, stop flow of gas. When heated to decomposition it emits acrid smoke and fumes.
Potential Exposure
Human Data.It is used as a raw material for butadiene, as a fuel forhousehold or industrial purposes (alone or in admixturewith propane). It is also used as an extractant, solvent, andaerosol propellant. It is used in plastic foam production as areplacement for fluorocarbons.
First aid
Move victim to fresh air. Call emergency medicalcare. Apply artificial respiration if victim is not breathing.Administer oxygen if breathing is difficult. Remove and isolate contaminated clothing and shoes. Clothing frozen to theskin should be thawed before being removed. In case of contact with liquefied gas, thaw frosted parts with lukewarmwater. Keep victim warm and quiet. Keep victim under observation.Ensure that medical personnel are aware of the material(s) involved and take precautions to protect themselves.
Source
Present in gasoline ranging from 4.31 to 5.02 vol % (quoted, Verschueren, 1983). Harley
et al. (2000) analyzed the headspace vapors of three grades of unleaded gasoline where ethanol
was added to replace methyl tert-butyl ether. The gasoline vapor concentrations of butane in the
headspace were 7.4 wt % for regular grade, 6.9 wt % for mid-grade, and 6.3 wt % for premium
grade.
Schauer et al. (1999) reported butane in a diesel-powered medium-duty truck exhaust at an
emission rate of 3,830 μg/km.
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of butane was 25.9 mg/kg of pine burned. Emission rates of butane were not measured during
the combustion of oak and eucalyptus.
California Phase II reformulated gasoline contained butane at a concentration of 7,620 mg/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 1,620 and 191,000 μg/km, respectively (Schauer et al., 2002).
Reported as an impurity (0.4 wt %) in 99.4 wt % trans-2-butene (Chevron Phillips, 2004).
Environmental Fate
Biological. In the presence of methane, Pseudomonas methanica degraded butane to 1-butanol,
methyl ethyl ether, butyric acid, and 2-butanone (Leadbetter and Foster, 1959). 2-Butanone was
also reported as a degradation product of butane by the microorganism Mycobacterium smegmatis
(Riser-Roberts, 1992). Butane may biodegrade in two ways. The first is the formation of butyl
hydroperoxide which decomposes to 1-butanol followed by oxidation to butyric acid. The other
pathway involves dehydrogenation yielding 1-butene, which may react with water forming 1-
butanol (Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer and
Finnerty, 1984). The most common degradative pathway involves the oxidation of the terminal methyl group forming the corresponding alcohol (1-butanol). The alcohol may undergo a series of
dehydrogenation steps forming butanal followed by oxidation forming butyric acid. The fatty acid
may then be metabolized by β-oxidation to form the mineralization products, carbon dioxide, and
water (Singer and Finnerty, 1984).
Photolytic. Major products reported from the photooxidation of butane with nitrogen oxides
under atmospheric conditions were acetaldehyde, formaldehyde, and 2-butanone. Minor products
included peroxyacyl nitrates and methyl, ethyl and propyl nitrates, carbon monoxide, and carbon
dioxide. Biacetyl, tert-butyl nitrate, ethanol, and acetone were reported as trace products
(Altshuller, 1983; Bufalini et al., 1971). The amount of sec-butyl nitrate formed was about twice
that of n-butyl nitrate. 2-Butanone was the major photooxidation product with a yield of 37%
(Evmorfopoulos and Glavas, 1998). Irradiation of butane in the presence of chlorine yielded
carbon monoxide, carbon dioxide, hydroperoxides, peroxyacid, and other carbonyl compounds
(Hanst and Gay, 1983). Nitrous acid vapor and butane in a “smog chamber” were irradiated with
UV light. Major oxidation products identified included 2-butanone, acetaldehyde, and butanal.
Minor products included peroxyacetyl nitrate, methyl nitrate, and unidentified compounds (Cox et
al., 1981).
The rate constant for the reaction of butane and OH radicals in the atmosphere at 300 K is 1.6 x
10-12 cm3/molecule?sec (Hendry and Kenley, 1979). Based upon a photooxidation rate constant of
2.54 x 10-12 cm3/molecule?sec with OH radicals in summer daylight, the atmospheric lifetime is 54
h (Altshuller, 1991). At atmospheric pressure and 298 K, Darnall et al. (1978) reported a rate
constant of 2.35–4.22 x 10-12 cm3/molecule?sec for the same reaction. A rate constant of 1.28 x
10-11 L/molecule?sec was reported for the reaction of butane with OH radicals in air at 298 K,
respectively (Greiner, 1970). At 296 K, a rate constant of 6.5 x 10-17 cm3/molecule?sec was
reported for the reaction of butane with NO3 (Atkinson, 1990).
Chemical/Physical. Complete combustion in air produces carbon dioxide and water. Butane will
not hydrolyze because it has no hydrolyzable functional group.
Solubility in organics
At 17 °C (mL/L): chloroform (25,000), ether (30,000) (Windholz et al., 1983). At 10 °C (mole
fraction): acetone (0.2276), aniline (0.04886), benzene (0.5904), 2-butanone (0.3885),
cyclohexane (0.6712), ethanol (0.1647), methanol (0.04457), 1-propanol (0.2346), 1-butanol
(0.2817). At 25 °C (mole fraction): acetone (0.1108), aniline (0.03241), benzene (0.2851), 2-
butanone (0.1824), cyclohexane (0.3962), ethanol (0.07825), methanol (0.03763), 1-propanol
(0.1138), 1-butanol (0.1401) (Miyano and Hayduk, 1986).
Mole fraction solubility in 1-butanol: 0.140, 0.0692, and 0.0397 at 25, 30, and 70 °C, respectively;
in chlorobenzene: 0.274, 0.129, and 0.0800 at 25, 30, and 70 °C, respectively, and in
octane: 0.423, 0.233, and 0.152 at 25, 30, and 70 °C, respectively (Hayduk et al., 1988).
Mole fraction solubility in 1-butanol: 0.139 and 0.0725 at 25 and 70 °C, respectively; in
chlorobenzene: 0.269 and 0.131 at 25 and 70 °C, respectively; and in carbon tetrachloride: 0.167
at 70 °C (Blais and Hayduk, 1983).
storage
Color Code—Red Stripe: Flammability Hazard:Store separately from all other flammable materials. Prior toworking with butane you should be trained on its properhandling and storage. All appropriate sections of theOSHA Standard 1910.111, Storage, Handling, of LiquefiedPetroleum Gases must be followed. Store in tightly closedcontainers in a cool, well-ventilated area away from incompatible materials listed above and heat. Metal containersinvolving the transfer of this chemical should be groundedand bonded. Drums must be equipped with self-closingvalves, pressure vacuum bungs, and flame arresters. Use onlynonsparking tools and equipment, especially when openingand closing containers of this chemical. Sources of ignition,such as smoking and open flames, are prohibited where thischemical is used, handled, or stored in a manner that couldcreate a potential fire or explosion hazard. Procedures for thehandling, use, and storage of cylinders should be in compliance with OSHA 1910.101 and 1910.169, as with the recommendations of the Compressed Gas Association.
Shipping
Butane requires a shipping label of“FLAMMABLE GAS.” Butane falls in Hazard Class 2.1with no Packing Group specified.
Purification Methods
Dry by passing over anhydrous Mg(ClO4)2 and molecular sieves type 4A. Air is removed by prolonged and frequent degassing at -107o. [Beilstein 1 IV 236.]
Incompatibilities
Strong bases, strong oxidizers (e.g.,nitrates & perchlorates), chlorine, fluorine, (nickelcarbonyl 1 oxygen).
n-Butane Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| ANHUI WITOP BIOTECH CO., LTD | +8615255079626 | eric@witopchemical.com | China | 23556 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9320 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 | info@fdachem.com | China | 7845 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 | chemwill_asia@126.com | CHINA | 23931 | 58 |