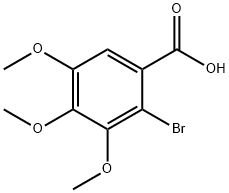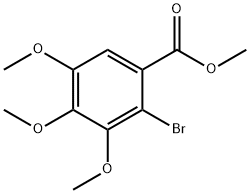
Methyl 2-broMo-3,4,5-triMethoxybenzoate synthesis
- Product Name:Methyl 2-broMo-3,4,5-triMethoxybenzoate
- CAS Number:1968-71-4
- Molecular formula:C11H13BrO5
- Molecular Weight:305.12
1916-07-0
446 suppliers
$6.00/25g

1968-71-4
5 suppliers
inquiry
Yield:1968-71-4 99%
Reaction Conditions:
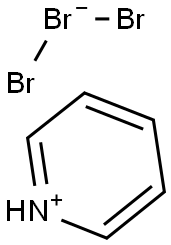
with NBS in acetonitrile at 20; for 16 h;Inert atmosphere;
Steps:
Methyl 2-bromo-3,4,5-trimethoxybenzoate (19)
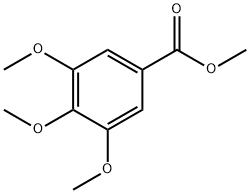
To a solution of methyl 3,4,5-trimethoxybenzoate (18) (1.13 g, 5 mmol) in acetonitrile (50 mL) was added N-bromosuccinimide (NBS) (0.89 g, 5 mmol). The resulting solution was stirred at ambient temperature for 16 h. After solvent removal, the residue was diluted with water (50 mL) and extracted with EtOAc (2 30 mL). The combined organic layers were washed successively with water (30 mL) and brine (20 mL), dried over anhydrous magnesium sulfate, filtered and concentrated. Purification by column chromatography (15% EtOAc/ hexane) afforded methyl 2-bromo-3,4,5-trimethoxybenzoate (19). Colorless syrup; yield: 1.51 g (99%); Rf = 0.36 (EtOAc/hexane, 1:4). IR (neat): 3033, 1722, 1586, 1430, 1311, 1202, 1050, 734 cm-1. 1H NMR (400 MHz, CDCl3): = 7.15 (s, 1H), 3.93 (s, 3 H), 3.92 (s, 3 H), 3.89 (s, 3 H), 3.88 (s, 3 H). 13C NMR (100 MHz, CDCl3): = 166.2, 152.1, 151.3, 145.8, 127.3, 109.9, 109.3, 61.0, 60.8, 56.1, 52.3. HRMS (ESI-TOF): m/z [M + Na]+ calcd for C11H13BrO5Na: 326.9844; found: 326.9832.
References:
Deelertpaiboon, Pramchai;Kongsriprapan, Sopanat;Krajangsri, Suppachai;Betterley, Nolan M.;Kuhakarn, Chutima;Reutrakul, Vichai [Synthesis,2022,vol. 54,# 13,p. 3093 - 3104]
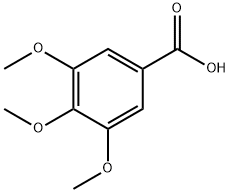
118-41-2
582 suppliers
$14.00/5g

1968-71-4
5 suppliers
inquiry
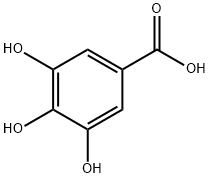
149-91-7
754 suppliers
$5.00/25g

1968-71-4
5 suppliers
inquiry