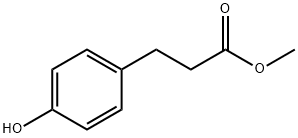
Methyl 3-(4-hydroxyphenyl)propionate synthesis
- Product Name:Methyl 3-(4-hydroxyphenyl)propionate
- CAS Number:5597-50-2
- Molecular formula:C10H12O3
- Molecular Weight:180.2
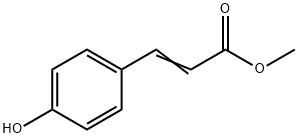
3943-97-3

5597-50-2
General procedure for the synthesis of methyl 4-hydroxyphenylpropionate from methyl 4-hydroxycinnamate: Pd/C (0.1 g) was added to a solution of methyl (E)-3-(4-hydroxyphenyl)acrylate (1.0 g, 5.6 mmol) in ethanol (20 mL). The reaction mixture was stirred vigorously at room temperature under hydrogen atmosphere (1 bar) for 21 hours. Upon completion of the reaction, the suspension was filtered through a diatomaceous earth pad and evaporated to dryness under vacuum to afford the oily product methyl 3-(4-hydroxyphenyl)propionate (1.0 g, 5.6 mmol, 99% yield). The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3): δ 7.07 (ddd, J=8.8,2.8,2.0 Hz, 2H), 6.76 (ddd, J=8.8,2.8,2.0 Hz, 2H), 4.72 (br s, 1H), 3.67 (s, 3H), 2.88 (t, J=7.8 Hz, 2H). 2.60 (t, J=7.8Hz, 2H).
Yield:5597-50-2 95.9%
Reaction Conditions:
Stage #1:4-hydroxyphenylpropionic acid with potassium carbonate in N,N-dimethyl-formamide at 20; for 0.5 h;
Stage #2:methyl iodide in N,N-dimethyl-formamide at 0 - 20; for 3 h;
Steps:
1
Intermediate 2: Methyl 3-(4-hydroxyphenyl) propanoateTo a 1000 mL RB flask fitted with magnetic stirrer was charged 250 mL of DMF, 3-(4- Hydroxy-phenyl)-propanoic acid (25.0 g, 150.43 mmol) and K2C03 (41.58 g, 300.87 mmol). The resulting mixture was stirred at RT for 30 minutes. Methyl Iodide (25.627 g, 180.51 mmol) was added to the resulting mixture which was precooled to 0 ¾. The resulting mixture was stirred at RT for 3 h. After completion of the reaction (reaction monitored by TLC), the solvent was removed under reduced pressure and the crude mass was dissolved in ethyl acetate (250 mL). The organic layer was washed with water (250 mL), saturated sodium bicarbonate solution (250 mL X 2), and saturated brine solution (250 mL). The organic layer was dried over anhydrous Na2S04 and the solvent was removed under reduced pressure. The product was obtained as yellow color oil (26 g, Yield: 95.9 %): MS (ESI, 120 eV) : m/z = 178.9 (M- H)+; 1H NMR (300MHz, CDCI3): δ 6.97-7.00(d, 2H), 6.66-6.69(d, 2H), 4.94(s, 1 H), 3.59(s, 3H), 2.78-2.83(t, 2H), 2.50-2.55(t, 2H).
References:
CONNEXIOS LIFE SCIENCES PVT. LTD.;RANGANATH RAO, Jagannath Madanahalli;ARUMUGAM, Nagarajan;ANSARI, Mohd Mudabbir;GUDLA, Chandrasekhar;PACHIYAPPAN, Shanmugam;RAMALINGAM, Manivannan;GEORGE, Jenson;ARUL, George Fernanda;BOMMEGOWDA, Y, Kenchegowda;ANGUPILLAI, Sathesh Kumar;KOTTAMALAI, Ramamoorthy;JIDUGU, Pradeep;RAO, D, Shivanageshwara WO2012/11125, 2012, A1 Location in patent:Page/Page column 59; 60

3943-97-3
168 suppliers
$6.00/1g

5597-50-2
253 suppliers
$5.00/1g
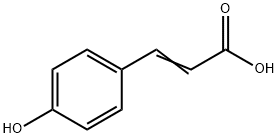
7400-08-0
460 suppliers
$29.00/10g

5597-50-2
253 suppliers
$5.00/1g
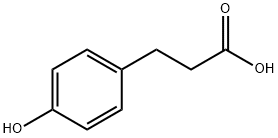
501-97-3
544 suppliers
$10.00/5g

5597-50-2
253 suppliers
$5.00/1g

