
Methyl-4-amino-3-fluorobenzoate synthesis
- Product Name:Methyl-4-amino-3-fluorobenzoate
- CAS Number:185629-32-7
- Molecular formula:C8H8FNO2
- Molecular Weight:169.15
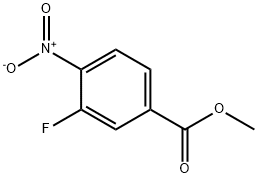
185629-31-6

185629-32-7
General procedure for the synthesis of methyl 4-amino-3-fluorobenzoate from methyl 3-fluoro-4-nitrobenzoate: Methyl 3-fluoro-4-nitrobenzoate (2.50 g, 12.5 mmol) and Pd/C catalyst (300 mg, 10 wt%) were placed in a 1:1 EtOAc/EtOH solvent mixture (40 mL). The reaction mixture was stirred at room temperature and 1 atm hydrogen atmosphere for 16 hours. After completion of the reaction, the mixture was filtered through diatomaceous earth and the filtrate was concentrated under reduced pressure to afford methyl 4-amino-3-fluorobenzoate (2.1 g, 99% yield) as a beige solid. Mass spectrometry analysis (ES+): the theoretical molecular weight of C8H8FNO2 was 169 and the measured value was 170 [M + H]+.

185629-31-6
171 suppliers
$5.00/250mg

185629-32-7
105 suppliers
$6.00/1g
Yield: 99%
Reaction Conditions:
with palladium on activated charcoal;hydrogen in ethanol;ethyl acetate at 20; under 760.051 Torr; for 16 h;
Steps:
135 Methyl 4-amino-3-fluorobenzoate
A mixture of methyl 3-fluoro-4-nitrobenzoate (2.50 g,12.5 mmol) and Pd/C (300 mg, 10 wt%) in 1:1 EtOAc/EtOH (40 mL) was stirred at rt for 16h under 1 atm of H2. The mixture was filtered through Celite and concentrated to give the titlecompound (2.1 g, 99%) as a beige solid. MS (ES+) C8H8FNO2 requires: 169, found: 170[M+Hj
References:
TESARO, INC.;JONES, Philip;CZAKO, Barbara;BURKE, Jason P.;CROSS, Jason;LEONARD, Paul Graham WO2018/81276, 2018, A1 Location in patent:Page/Page column 154

67-56-1
790 suppliers
$7.29/5ml-f
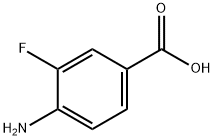
455-87-8
218 suppliers
$6.00/1g

185629-32-7
105 suppliers
$6.00/1g

185629-31-6
171 suppliers
$5.00/250mg
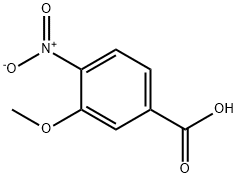
5081-36-7
223 suppliers
$6.00/1g
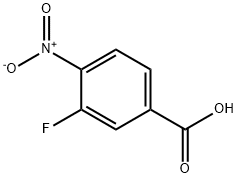
403-21-4
258 suppliers
$6.00/1g

185629-32-7
105 suppliers
$6.00/1g
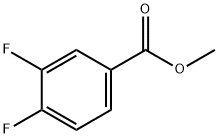
369-25-5
170 suppliers
$13.00/25g

185629-32-7
105 suppliers
$6.00/1g

403-21-4
258 suppliers
$6.00/1g

185629-32-7
105 suppliers
$6.00/1g