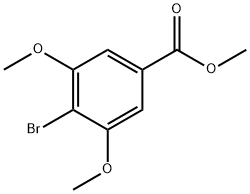
methyl 4-bromo-3,5-dimethoxybenzoate synthesis
- Product Name:methyl 4-bromo-3,5-dimethoxybenzoate
- CAS Number:26050-64-6
- Molecular formula:C10H11BrO4
- Molecular Weight:275.1

67-56-1
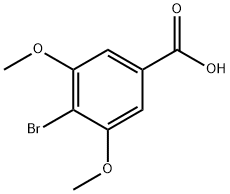
56518-42-4

26050-64-6
To a round bottom flask was added 4-bromo-3,5-dimethoxybenzoic acid (Compound 8, 4 g, 15.3 mmol) and anhydrous methanol (100 mL). The reaction mixture was cooled to 0 °C and thionyl chloride (11.1 mL, 153 mmol) was added slowly and dropwise with stirring. After the dropwise addition was completed, the reaction system was heated to 80 °C and the reaction was stirred at this temperature for 2 hours. Upon completion of the reaction, the reaction mixture was concentrated under reduced pressure to remove the solvent. The residue was dissolved in ethyl acetate (100 mL) and the organic layer was washed sequentially with water (50 mL x 2) and saturated saline (50 mL). The organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to afford methyl 4-bromo-3,5-dimethoxybenzoate as a white crystalline solid (Compound 12, 4.24 g, quantitative yield).Results of LC-MS analysis: retention time (TR) = 2.3 min; [M+H]+ = 274.9, 277.0 (bromine isotope peak).
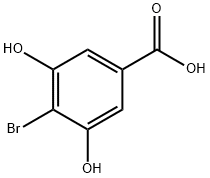
16534-12-6
215 suppliers
$21.21/1gm:

26050-64-6
44 suppliers
$5.00/1g
Yield: 92%
Reaction Conditions:
with potassium carbonate;dimethyl sulfate in acetone for 24 h;Reflux;Inert atmosphere;
Steps:
1 4.1.1 Methyl 4-bromo-3,5-dimethoxybenzoate (4)
To the stirred solution of 4-bromo-3,5-dihydroxybenzoic acid (3) 24.0 g, 0.103 mol) in dry acetone (100 mL), K2CO3 (20.0 g, 0.145 mol), and dimethyl sulfate (20 mL, 0.309 mol) was added with caution.
The reaction mixture was refluxed for 24 h.
On completion of reaction (TLC control) the reaction mixture was filtered off and the filtrate was concentrated in vacuo.
Recrystallization from ethanol afforded methyl 4-bromo-3,5-dimethoxybenzoate 4 as colorless prisms. (24.5 g, 92%) Rf=0.65; Mp 124-126 °C (lit.
References:
Saeed, Aamer;Mahesar, Parvez Ali [Tetrahedron,2014,vol. 70,# 7,p. 1401 - 1407]

67-56-1
790 suppliers
$7.29/5ml-f

56518-42-4
148 suppliers
$14.00/1g

26050-64-6
44 suppliers
$5.00/1g
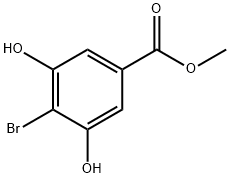
34126-16-4
37 suppliers
$55.60/2 g

74-88-4
356 suppliers
$15.00/10g

26050-64-6
44 suppliers
$5.00/1g

16534-12-6
215 suppliers
$21.21/1gm:

77-78-1
319 suppliers
$19.69/1ml

26050-64-6
44 suppliers
$5.00/1g

74-88-4
356 suppliers
$15.00/10g

26050-64-6
44 suppliers
$5.00/1g