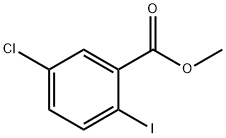
METHYL 5-CHLORO-2-IODOBENZOATE synthesis
- Product Name:METHYL 5-CHLORO-2-IODOBENZOATE
- CAS Number:289039-82-3
- Molecular formula:C8H6ClIO2
- Molecular Weight:296.49

67-56-1
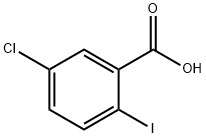
13421-00-6

289039-82-3
General procedure for the synthesis of methyl 5-chloro-2-iodobenzoate from methanol and 5-chloro-2-iodobenzoic acid: a mixture of 5-chloro-2-iodobenzoic acid (3.0 g, 10.62 mmol), dichlorosulfoxide (12 mL), and N,N-dimethylformamide (0.6 mL) was gently heated with a heat gun until a homogeneous solution was formed (about 15 minutes). Subsequently, the reaction solution was continued to be stirred at 23 °C for 30 min, after which the solvent was removed by concentration under reduced pressure. Methanol (24 mL) was added to the crude product and stirred at 23 °C for 30 min. After the reaction was completed, the reaction solution was again concentrated under reduced pressure. The crude product was purified by rapid chromatography on a Biotage silica gel column with a gradient of cyclohexane to cyclohexane:ethyl acetate (85:15) as eluent to give methyl 5-chloro-2-iodobenzoate (3.02 g, 10.20 mmol, 96% yield).UPLC-MS analysis showed a retention time of 1.17 min and a molecular ion peak m/z 296.6 [M + H]+ , analyzed by method 12.

13421-00-6
139 suppliers
$10.00/5g

74-88-4
357 suppliers
$15.00/10g

289039-82-3
82 suppliers
$6.00/100mg
Yield:289039-82-3 98%
Reaction Conditions:
with sodium hydrogencarbonate in N,N-dimethyl-formamide at 20; for 24 h;
Steps:
1 Methyl 5-chloro-2-iodobenzoate.
To a round bottom flask was added NaHCO3 (22.3 g, 266 mmol), 5-chloro-2-iodobenzoic acid (25.0 g, 89.0 mmol), DMF (88 mL), and Mel (11.1 mL, 177 mmol) and the reaction was stirred at room temperature. After 24 h, the reaction was partitioned between water and EtOAc. The aqueous phase was extracted with EtOAc (2x). The combined organic extracts were washed with water (3x), brine, dried over Na2SO4, and concentrated. The crude residue was purified via Biotage to afford methyl 5-chloro-2-iodobenzoate (25.8 g, 87.0 mmol, 98% yield). LC/MS m/z 297 [M+H]+
References:
WO2013/184876,2013,A1 Location in patent:Paragraph 00144; 00151; 00152

67-56-1
790 suppliers
$9.00/25ml

13421-00-6
139 suppliers
$10.00/5g

289039-82-3
82 suppliers
$6.00/100mg