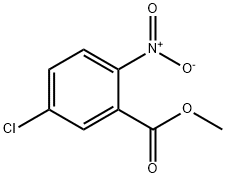
Methyl 5-chloro-2-nitrobenzoate synthesis
- Product Name:Methyl 5-chloro-2-nitrobenzoate
- CAS Number:51282-49-6
- Molecular formula:C8H6ClNO4
- Molecular Weight:215.59
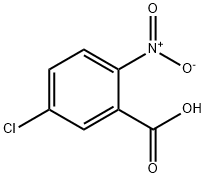
2516-95-2

74-88-4

51282-49-6
This embodiment relates to the synthesis of methyl 2-nitro-5-chlorobenzoate: to a stirred solution of 5-chloro-2-nitrobenzoic acid (9.393 g, 46.60 mmol) in anhydrous DMF (155 mL) at 0 °C was added potassium carbonate (K2CO3, 13.23 g, 95.72 mmol) and iodomethane (MeI, 19.38 g, 8.5 mL. 136.5 mmol), followed by slow warming of the reaction mixture to 40 °C. After 1 h of reaction, the solution was cooled to room temperature and diluted with ethyl acetate (EtOAc, 115 mL). The organic phase was washed sequentially with water (3 x 100 mL) and saturated sodium chloride solution (3 x 100 mL). The combined organic phases were dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure, and the resulting crude product was purified by silica gel column chromatography with 40-60% ethyl acetate/hexane as eluent to afford methyl 2-nitro-5-chlorobenzoate (9.244 g, 42.87 mmol, 92% yield) as a light yellow solid. The structure of the product was confirmed by 1H NMR (400 MHz, CDCl3) and 13C NMR (100 MHz, CDCl3).

67-56-1
790 suppliers
$7.29/5ml-f

2516-95-2
323 suppliers
$5.00/5g

51282-49-6
175 suppliers
$13.00/5g
Yield: 98.2%
Reaction Conditions:
with thionyl chloride at 80; for 4 h;Cooling with ice;Reflux;
Steps:
methyl 5-chloro-2-nitrobenzoate (4)
To a stirred solution of 5-chloro-2-nitrobenzoic acid 3 (4.02 g, 20 mmol) in 125 ml of methanol, under ice-cooling, was added thionyl chloride (10 ml, 150 mmol) dropwise over 10 minutes. Then the solution was heated to 80, and kept refluxing for about 4 h. Methanol is distilled out and 50 ml of water is added. The separated ester was extracted with EtOAc and washed with 50 ml of saturated sodium bicarbonate solution. Drying (Na2SO4) and evaporation of the EtOAc gave the white solid (4.0 g, 93%). HPLC purity 98.2%; 1H NMR (300 MHz, DMSO-d6) δ 8.14 (dd, J = 8.6, 1.8 Hz, 1H), 7.98 (d, J = 2.3 Hz, 1H), 7.94 - 7.88 (m, 1H), 3.87 (d, J = 1.5 Hz, 3H); ESI-MS m/z: 216.2 [M+H]+.
References:
Geng, Aixin;Cui, Hao;Zhang, Liyuan;Chen, Xin;Li, Hongmei;Lu, Tao;Zhu, Yong [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 13,p. 1605 - 1608] Location in patent:supporting information

2516-95-2
323 suppliers
$5.00/5g

74-88-4
356 suppliers
$15.00/10g

51282-49-6
175 suppliers
$13.00/5g

2516-95-2
323 suppliers
$5.00/5g

77-78-1
319 suppliers
$19.69/1ml

51282-49-6
175 suppliers
$13.00/5g
2905-65-9
150 suppliers
$14.00/5g

51282-49-6
175 suppliers
$13.00/5g

2516-95-2
323 suppliers
$5.00/5g

51282-49-6
175 suppliers
$13.00/5g