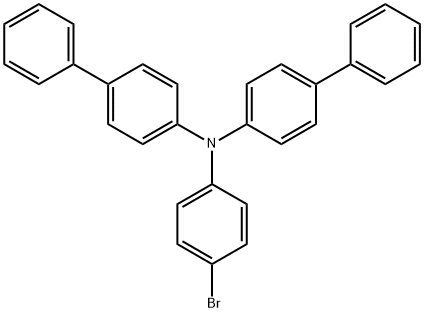
N-(4-BroMophenyl)-N,N-bis(1,1'-biphenyl-4-yl)aMine synthesis
- Product Name:N-(4-BroMophenyl)-N,N-bis(1,1'-biphenyl-4-yl)aMine
- CAS Number:499128-71-1
- Molecular formula:C30H22BrN
- Molecular Weight:476.41
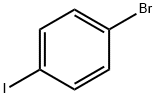
589-87-7
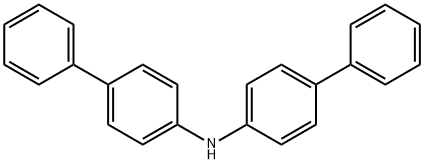
102113-98-4

499128-71-1
The general procedure for the synthesis of N-(4-bromophenyl)-N,N-bis(1,1'-biphenyl-4-yl)amine from p-bromoiodobenzene and bis(4-biphenyl)amine was as follows: bis(4-biphenyl)amine (23.7 g, 73.8 mmol) was added to a round-bottomed flask, followed by the addition of p-bromoiodobenzene (20.9 g, 73.8 mmol), sodium tert-butanolate (10.7 g, 111 mmol), p-butylpropanol (0.33 g, 1.47 mmol), and ultrasonically deoxygenated toluene (1.5 L). 111 mmol), palladium acetate (0.33 g, 1.47 mmol), and ultrasonically deoxygenated toluene (1.5 L). The mixture was heated to reflux overnight under nitrogen protection. After completion of the reaction, the reaction solution was cooled and extracted with ethyl acetate and water. The organic layer was separated and dried with anhydrous magnesium sulfate. The solvent was removed by distillation under reduced pressure to give the crude product. The crude product was purified by column chromatography using silica gel as stationary phase and dichloromethane/hexane as eluent to afford the target product N-(4-bromophenyl)-N,N-bis(1,1'-biphenyl-4-yl)amine (28.8 g, 82% yield).

589-87-7
540 suppliers
$10.00/1g

102113-98-4
309 suppliers
$6.00/1g

499128-71-1
167 suppliers
$7.00/100mg
Yield:499128-71-1 94%
Reaction Conditions:
with 1,10-Phenanthroline;potassium hydroxide;copper dichloride in toluene for 12 h;
Steps:
10.2; 11.2
(2) 30.53g (0.095mol) of bis([1,1'-biphenyl]-4-yl)amine (MW: 321.42),P-bromoiodobenzene (MW: 282.91) 26.88g (0.095mol),Cuprous chloride (MW: 99.00) 6.02g (0.0608mol),1,10-phenanthroline (MW: 180.2) 6.16g (0.0342mol),Potassium hydroxide (MW: 56) 31.92g (0.57mol) and toluene 530mL,Put it in a 1L reaction flask,Backflow diversion,Reaction for 12h; after the reaction is over,Cool down,filter,Wash with water to neutral, remove solvent,Dry to obtain N-([1,1'-biphenyl]-4-yl)-N-(4-bromophenyl)-[1,1'-biphenyl]-4-amine (MW: 476.42) 42.54g (0.0893mol), yield 94%;
References:
Yantai Xianhua Optoelectric Materials Institute Co., Ltd.;Sun Wei;Feng Peichuan;Chen Yue;Hu Lingfeng;Chen Yili;Wei Peng;Yang Yang CN111484468, 2020, A Location in patent:Paragraph 0105-0108; 0110; 0113-0116; 0118
![N-[1,1-biphenyl]-4-yl-N-phenyl-[1,1-Biphenyl]-4-amine](/CAS/20180703/GIF/122215-84-3.gif)
122215-84-3
44 suppliers
$28.00/250mg

499128-71-1
167 suppliers
$7.00/100mg
106-37-6
479 suppliers
$9.00/5g

102113-98-4
309 suppliers
$6.00/1g

499128-71-1
167 suppliers
$7.00/100mg
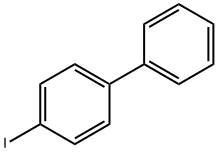
1591-31-7
333 suppliers
$8.00/5g

106-40-1
483 suppliers
$12.00/5g

499128-71-1
167 suppliers
$7.00/100mg
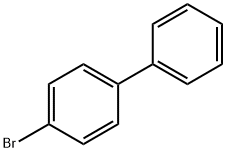
92-66-0
578 suppliers
$10.00/1g

106-40-1
483 suppliers
$12.00/5g

499128-71-1
167 suppliers
$7.00/100mg