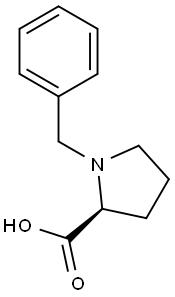
N-BENZYL-L-PROLINE synthesis
- Product Name:N-BENZYL-L-PROLINE
- CAS Number:31795-93-4
- Molecular formula:C12H15NO2
- Molecular Weight:205.25

100-44-7
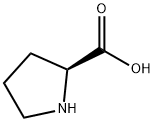
147-85-3

31795-93-4
L-proline (5.00 g, 43.44 mmol, 1.0 eq.) and potassium hydroxide (9.78 g, 174.3 mmol, 4 eq.) were dissolved in isopropanol (50 mL) and stirred at 40 °C until the solution was clarified. Subsequently, benzyl chloride (8.25 g, 7.50 mL, 65.2 mmol, 1.5 eq.) was added and the reaction continued to be stirred at 40 °C for 6 hours. Upon completion of the reaction, the reaction solution was neutralized with concentrated aqueous hydrochloric acid to pH 5-6. After neutralization, chloroform (30 mL) was added and the mixture was stirred overnight. The resulting precipitate was removed by filtration and the precipitate was washed with chloroform (30 mL). The organic phases were combined and the solvent was evaporated under vacuum. The resulting residue was treated with acetone (30 mL) to precipitate the crude product, which was filtered and washed with acetone to afford the white solid product (S)-1-benzylpyrrolidine-2-carboxylic acid (5.39 g, 26.26 mmol, 60% yield). The product was characterized as follows: Rf 0.09 (dichloromethane:methanol = 9:1); melting point 175 °C (literature value 167 °C); νmax(neat)/ cm-1 3041, 2992, 2969, 1634, 1450, 1375, 1311, 1190, 753, 704; 1H NMR (300 MHz, D2O) δ 7.53 (s, br, 5H, Ar-H), 4.40 (s, 2H, CH2Ph), 4.01 (dd, 1H, J = 6.7, 9.3 Hz, CHCO2H), 3.73-3.58 (m, 1H, CH2CH2aN), 3.38-3.21 (m, 1H, CH2CH2bN), 2.62-2.41 (m, 1H, CH2aCH), 2.27-1.89 (m, 3H, CH2bCH and CH2CH2N); 13C NMR (75 MHz, D2O) δ 173.54, 130.55, 130.05, 129.96, 129.25, 68.24, 58.30, 54.61, 28.79, 22.78; HRMS m/z ( ESI) 206.1215 ([M + H]+, calculated value 206.1165).

100-39-0
429 suppliers
$10.00/10 g

147-85-3
1060 suppliers
$6.00/25g

31795-93-4
146 suppliers
$9.00/250mg
Yield: 92%
Reaction Conditions:
Stage #1:benzyl bromide;L-proline with potassium hydroxide in isopropyl alcohol at 40;
Stage #2: with hydrogenchloride in chloroform;water;isopropyl alcohol; pH=4 - 5 at 20; for 12 h;
Steps:
3.1.1. General Procedure for the Preparation of Compounds 6
General procedure: To a solution of proline (2.0 g, 17 mmol) and KOH (2.85 g, 51 mmol) in i-PrOH (50 mL),4-substituted benzyl derivative (21 mmol) was added at 40° C. Afterwards, the mixture was stirred for8 h at 40° C and then the reaction mixture was cooled to room temperature. 6 M HCl was added toadjust the pH value of the mixture to 4-5 and then CHCl3 (50 mL) was added. The mixture was stirredfor 12 h, followed by filtration and evaporation in vacuo. The residue was purified by recrystallizationin acetone at 0° C to give 6 (about 90% yield).
References:
Yao, Yangyang;Li, Renze;Liu, Xiaoyu;Yang, Feilong;Yang, Ying;Li, Xiaoyu;Shi, Xiang;Yuan, Tianyi;Fang, Lianhua;Du, Guanhua;Jiao, Xiaozhen;Xie, Ping [Molecules,2017,vol. 22,# 10,art. no. 1766]

100-44-7
658 suppliers
$13.50/250G

147-85-3
1060 suppliers
$6.00/25g

31795-93-4
146 suppliers
$9.00/250mg

147-85-3
1060 suppliers
$6.00/25g

100-51-6
1432 suppliers
$5.00/100g

31795-93-4
146 suppliers
$9.00/250mg

100-52-7
971 suppliers
$17.30/2g

147-85-3
1060 suppliers
$6.00/25g

31795-93-4
146 suppliers
$9.00/250mg
83528-04-5
0 suppliers
inquiry

31795-93-4
146 suppliers
$9.00/250mg