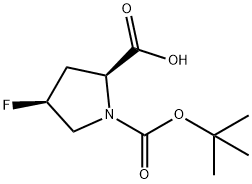
N-BOC-cis-4-fluoro-L-proline synthesis
- Product Name:N-BOC-cis-4-fluoro-L-proline
- CAS Number:203866-13-1
- Molecular formula:C10H16FNO4
- Molecular Weight:233.24
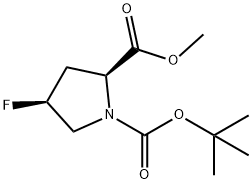
203866-16-4

203866-13-1
General procedure for the synthesis of (2S,4S)-4-fluoro-1-tert-butoxycarbonylpyrrolidine-2-carboxylic acid from N-tert-butoxycarbonyl-cis-4-fluoro-L-proline methyl ester: To a solution of N-tert-butoxycarbonyl-cis-4-fluoro-L-proline methyl ester (5.83 g, 23.58 mmol) in tetrahydrofuran (THF, 30 mL) was added, at 0 °C, lithium hydroxide (LiOH, 1.98 g) in aqueous solution (30 mL). The reaction mixture was stirred at room temperature for 2 h. The pH was subsequently adjusted to 5 with dilute hydrochloric acid (1 M). After evaporating THF under reduced pressure, the pH of the aqueous layer was adjusted to 2 with dilute hydrochloric acid (1 M) and extracted with ethyl acetate (EtOAc, 80 mL x 3). The organic layers were combined, dried over anhydrous sodium sulfate (Na2SO4) and concentrated under reduced pressure to afford (2S,4S)-4-fluoro-1-tert-butoxycarbonylpyrrolidine-2-carboxylic acid as a white solid (5.3 g, 96% yield). The structure of the compound was confirmed by the following spectral data: mass spectrum (ESI, positive ion mode) m/z: 234.24 [M + H]+; NMR hydrogen spectrum (400 MHz, CDCl3) δ (ppm): 8.76 (broad single peak, 1H), 5.28-5.12 (multiple peaks, 1H), 4.56-4.44 (multiple peaks, 1H), 3.86- 3.58 (multiple peaks, 2H), 2.77-2.01 (multiple peaks, 2H), 1.48-1.44 (double peaks, 9H, J = 16 Hz).

203866-16-4
115 suppliers
$40.00/250mg

203866-13-1
225 suppliers
$6.00/250mg
Yield:203866-13-1 96%
Reaction Conditions:
Stage #1:1-tert-butyl 2-methyl (2S,4S)-4-fluoropyrrolidine-1,2-dicarboxylate with water;lithium hydroxide in tetrahydrofuran at 0 - 20; for 2 h;
Stage #2: with hydrogenchloride in water; pH=5
Steps:
13.2 Step 2) the preparation of compound 13-3
To a solution of compound 13-2 (5.83 g, 23.58 mmol) in THF (30 mL) at 0 °C was added LiOH aqueous solution (1.98 g, 30 mL), and the mixture was stirred at rt for 2 hrs and adjusted to pH 5 with diluted hydrochloric acid (1 M). The solvent THF was removed in vacuo, and the aqueous layer was adjusted to pH 2 with diluted hydrochloric acid (1 M) and extracted with EtOAc (80 mL x 3). The combined organic layers were dried over Na2S04 and concentrated in vacuo to give the title compound as a white solid (5.3 g, 96%). The compound was characterized by the following spectroscopic data: MS (ESI, pos.ion) m/z: 234.24 [M+H] +; NMR (400 MHz, CDC13) δ (ppm): 8.76 (brs, 1 H), 5.28-5.12 (m, 1 H), 4.56-4.44 (m, 1H), 3.86-3.58 (m, 2H). 2.77-2.01 (m. 2H), 1.48-1.44 (d, 9H, J = 16 Hz).
References:
SUNSHINE LAKE PHARMA CO., LTD.;ZHANG, Yingjun;ZHANG, Jiancun;XIE, Hongming;REN, Qingyun;TAN, Yumei;LUO, Huichao WO2014/19344, 2014, A1 Location in patent:Paragraph 00435
74844-91-0
417 suppliers
$5.00/5g

203866-13-1
225 suppliers
$6.00/250mg
587888-04-8
1 suppliers
inquiry

203866-13-1
225 suppliers
$6.00/250mg

89813-47-8
55 suppliers
$44.60/250mg

203866-13-1
225 suppliers
$6.00/250mg

24424-99-5
871 suppliers
$13.50/25G

203866-13-1
225 suppliers
$6.00/250mg