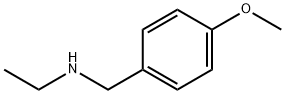
N-Ethyl-4-methoxybenzylamine synthesis
- Product Name:N-Ethyl-4-methoxybenzylamine
- CAS Number:22993-76-6
- Molecular formula:C10H15NO
- Molecular Weight:165.23
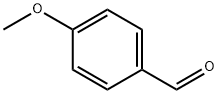
123-11-5
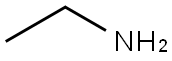
75-04-7
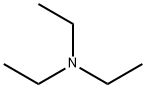
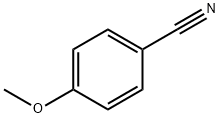
121-44-8

22993-76-6
N-Ethyl-4-methoxybenzylamine was synthesized as follows: anisaldehyde (15.6 g, 115 mmol) was mixed with ethylamine (2.0 M solution of THF, 87 mL, 174 mmol) in 1,2-dichloroethane (450 mL) under the protection of nitrogen, followed by the addition of glacial acetic acid (10.0 mL, 174 mmol). The reaction mixture was stirred at room temperature for 30 min and then cooled to 0 °C with an ice bath. Sodium triacetoxyborohydride (NaBH(OAc)3, 36.9 g, 174 mmol) was added in batches, after which the reaction mixture was stirred at room temperature overnight. After completion of the reaction, the mixture was concentrated and the residue was diluted with an alkaline solution (10 g NaOH dissolved in 100 mL of water) to a slightly alkaline color. The aqueous layer was extracted with ether and the organic phases were combined, washed sequentially with water and brine, dried and concentrated. The residue was purified by silica gel column chromatography, eluting first with dichloromethane solution containing 5% methanol, then with dichloromethane solution containing 4% triethylamine in 50% methanol, to afford the target product N-ethyl-4-methoxybenzylamine (11.2 g, 59% yield) as an oil.
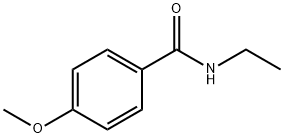
7403-41-0
18 suppliers
$57.00/250mg

22993-76-6
93 suppliers
$24.00/1g
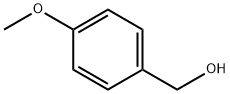
105-13-5
566 suppliers
$5.00/5G
Yield:22993-76-6 92 %Spectr. ,105-13-5 7 %Spectr.
Reaction Conditions:
with 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane in neat (no solvent) at 120; for 24 h;Inert atmosphere;Sealed tube;
Steps:
General procedure for hydroboration of secondary and tertiary amides
General procedure: N,N-dimethylbenzamide (0.0746 g,0.5 mmol), or N-methylbenzamide (0.0676 g, 0.5 mmol) was taken in culture tube (11 ml) at 25 °C, sealed with septum, the air in culture tube was replaced with argon gas. After adding pinacolborane (0.36 ml, 2.5 mmol 5 equiv), the screw cap was closed, stirred at 120 °C for 24 h. Afterthis time, it was cooled to room temperature, excess HBpin was quenched by adding water (0.1 ml). After adding 2 ml of 5 N NaOH (aq), reaction mixture was extracted with ether (20 ml). The organic part was dried over MgSO4, filtered and dried under high vacuum to remove volatile organic impurities. The yield was analyzed by 1HNMR (using acetonitrile [8μl, 0.33 eq] as an internal standard).
References:
Yi, Jaeeun;Kim, Hyun Tae;Jaladi, Ashok Kumar;An, Duk Keun [Bulletin of the Korean Chemical Society,2022,vol. 43,# 1,p. 129 - 132]
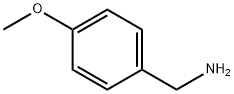
2393-23-9
474 suppliers
$20.00/25g

75-05-8
1049 suppliers
$10.00/10g

22993-76-6
93 suppliers
$24.00/1g

7403-41-0
18 suppliers
$57.00/250mg

22993-76-6
93 suppliers
$24.00/1g

2393-23-9
474 suppliers
$20.00/25g

22993-76-6
93 suppliers
$24.00/1g