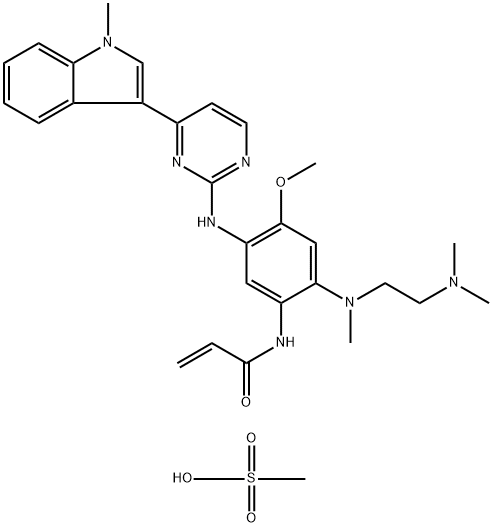
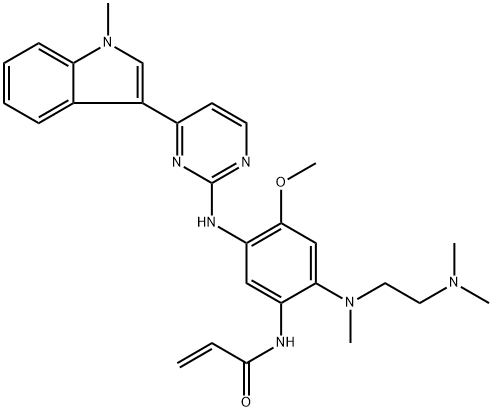
Osimertinib mesylate synthesis
- Product Name:Osimertinib mesylate
- CAS Number:1421373-66-1
- Molecular formula:C29H37N7O5S
- Molecular Weight:595.72
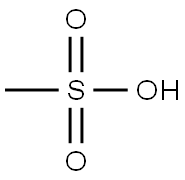
75-75-2
651 suppliers
$10.00/5g
1421373-65-0
384 suppliers
$28.00/1mg

1421373-66-1
383 suppliers
inquiry
Yield:1421373-66-1 96.73%
Reaction Conditions:
Stage #1: N-(2-{[2-(dimethylamino)ethyl](methyl)amino}-4-methoxy-5-{[4-(1-methyl-1H-indol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamidewith pyrographite in propan-2-one at 25 - 55;
Stage #2: methanesulfonic acid at 25 - 55;
Steps:
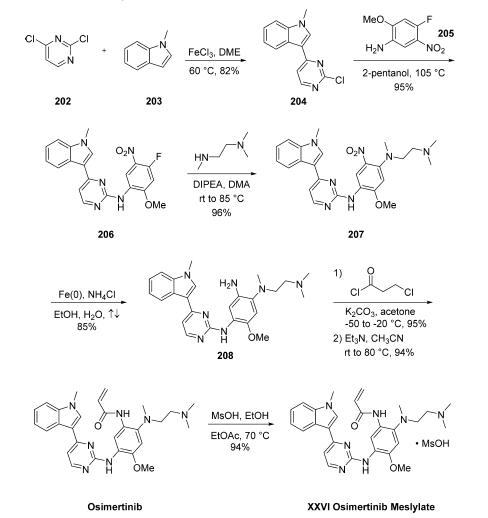
2 Example-2: Preparation of Osimertinib mesylate
Added the Osimertinib (75 g) fallowed by acetone (750 ml) in to a 1.0 L multi neck RB flask equipped with mechanical stirrer, thermometer socket and reflux condenser at 25-30°C the heated the reaction mixture to 50-55°C then maintained about 30 min. Thereafter, added activated carbon (2.5 g) to the above solution at 50-55°C, maintained abovel5-20 min, filtered the hot solution over hyflo bed and washed with acetone (100 ml).Collected the hot filtrate and washings transferred in to another 1.0 L multi-neck RB flask equipped with mechanical stirrer, thermometer socket and reflux condenser at above 35°C. Added the DM water (25 ml) then heated the reaction mixture to 50-55°C and maintained about 30 min. after that added the methane sulfonic acid (14.4 g) to the above reaction mass at 50-55°C in about lOmin. Maintained at 50-55°C for about 3-3.5 h, and cooled to 25-30°C in about 1.5-1.75h then filtered the product, washed with acetone (125 ml) and suck dried for about 15 min. product dried under vacuum at 80°C for 8-10 hr. Yield : 86.5 g; theory: 96.73%*HPLC purity: 99.84 %. IMP- A: 0.00%, IMP-B: 0.02%, IMP-C: 0.00%, IMP-D: 0.02%, IMP-E: 0.02%, IMP-F: 0.04%
References:
WO2021/111462,2021,A1 Location in patent:Paragraph 6; 7

75-75-2
651 suppliers
$10.00/5g
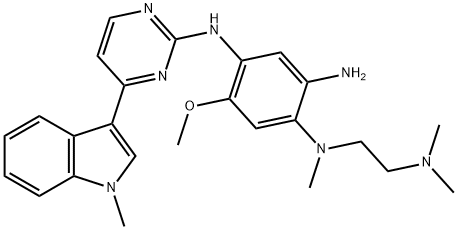
1421372-66-8
208 suppliers
inquiry
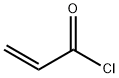
814-68-6
396 suppliers
$21.21/5gm:

1421373-66-1
383 suppliers
inquiry
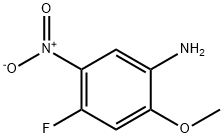
1075705-01-9
349 suppliers
$8.00/1g

1421373-66-1
383 suppliers
inquiry
![1,4-BenzenediaMine, N1-[2-(diMethylaMino)ethyl]-5-Methoxy-N1-Methyl-N4-[4-(1-Methyl-1H-indol-3-yl)-2-pyriMidinyl]-2-nitro-](/CAS/20150408/GIF/1421372-67-9.gif)
1421372-67-9
170 suppliers
inquiry

1421373-66-1
383 suppliers
inquiry
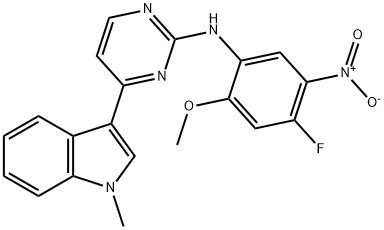
1421372-94-2
235 suppliers
inquiry

1421373-66-1
383 suppliers
inquiry