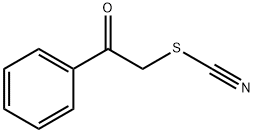
PHENACYL THIOCYANATE synthesis
- Product Name:PHENACYL THIOCYANATE
- CAS Number:5399-30-4
- Molecular formula:C9H7NOS
- Molecular Weight:177.22
Yield: 99%
Reaction Conditions:
Stage #1:ammonium thiocyanate with montmorillonite K10 clay in acetone at 20; for 0.666667 h;
Stage #2:1-phenyl-2-(p-tolylsulfonyloxy)ethanone at 20; for 0.00833333 h;Neat (no solvent);Grinding;
Steps:
General procedure for the preparation of K10-montmorillonite clay supported ammonium thiocyanate; General procedure for the synthesis of α-oxo thiocyanates by using K10-montmorillonite clay supported ammonium thiocyanate under catalyst and solvent free conditions
In a round bottomed 500 ml flask equipped with stir bar and containing 100 ml acetone, was added 7.6 g of ammonium thiocyanate salt and stirred at room temperature until the complete dissolution of salt. To this clear solution, 10 g of montmorillonite K10 clay was added in portions over 10 min with stirring. After complete addition, the formation of reddish suspension was observed which was vigorously stirred for another 30 min at room temperature. Then the suspension is placed in a rotary vacuum evaporator and the solvent was removed under reduced pressure. The dry solid crust adhering to the walls of the flask was flaked off with a spatula, and solvent evaporation was resumed. After complete drying, yielded, about 17.6 g of clay supported ammonium thiocyanate as a light red free flowing powder which shows no loss of reactivity after standing in an open powder box for one week.; Phenacyl bromide (1 mmol) and K10-montmorillonite clay supported ammonium thiocyanate (3 mmol) were taken in mortar, mixed with spatula, and ground with pestle for stipulated time (see Table 3). After complete conversion as indicated by TLC, the solid reaction mixture was directly loaded on silica gel column by avoiding aqueous work up-extraction step. Later elution with ethyl acetate-hexane (9:1-3:1) solvent system and evaporation of solvents in rotary vacuum evaporator afforded pure phenacyl thiocyanate (99%). Same procedure as discussed above was followed to prepare all thiocyanate compounds shown in this work. All compounds prepared were characterized by IR, Mass, and NMR spectroscopy.
References:
Meshram;Thakur, Pramod B.;Madhu Babu;Bangade, Vikas M. [Tetrahedron Letters,2012,vol. 53,# 14,p. 1780 - 1785] Location in patent:experimental part
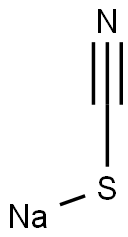
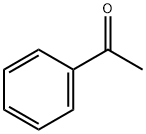
540-72-7
473 suppliers
$13.50/100G
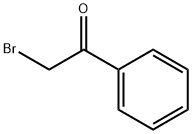
70-11-1
298 suppliers
$10.00/25g

5399-30-4
46 suppliers
$30.00/1 g
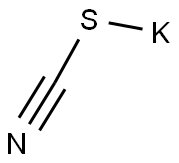
333-20-0
464 suppliers
$13.50/100G

70-11-1
298 suppliers
$10.00/25g

5399-30-4
46 suppliers
$30.00/1 g
![Ethanone, 2-[[(4-methylphenyl)sulfonyl]oxy]-1-phenyl-](/CAS/20180601/GIF/7257-94-5.gif)
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)