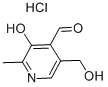
Pyridoxal hydrochloride synthesis
- Product Name:Pyridoxal hydrochloride
- CAS Number:65-22-5
- Molecular formula:C8H10ClNO3
- Molecular Weight:203.62
In addition, get about 5mL reaction solution and directly go up silicagel column, eluent is methylene dichloride: methyl alcohol=20: 1, obtain final elutriant through gradient elution, and be concentrated into dryly, can obtain white solidpyridoxalhydrochloride, fusing point 164.1-164.8 DEG C, nuclear-magnetism characterizes as follows: 1hNMR (400MHz, DMSO), δ: 8.27 (s, 1H, CHO), 6.63 (d, 1H, CH), 5.00-5.16 (m, 2H, CH2), 2.62 (s, 3H, CH3).
Because pyridoxalhydrochloride is unstable, difficult separation, reaction solution then adds p-ethoxyaniline 1.37g (10mmol), treated yellow Schiff alkali (IX) 2.10g that obtains without separating.The total recovery of two-step reaction is 88%.
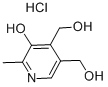
58-56-0
630 suppliers
$5.00/100mg

65-22-5
371 suppliers
$9.00/1g
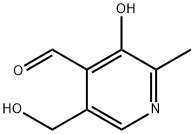
Yield:65-22-5 88 g
Reaction Conditions:
with hydrogenchloride;manganese(IV) oxide in water at 55; for 28 h;Temperature;
Steps:
2.1 Step 1. Preparation of pyridoxal hydrochloride
100 g of pyridoxine hydrochloride was dissolved in 1000 mL of 15% hydrochloric acid and 200 g of active was added.Manganese dioxide, stirred and heated to 55°C for reaction. After 28 hours of reaction, the raw material was completely converted and naturally cooled to room temperature 20-25.°C, the filtrate was filtered by vacuum filtration, and the filtrate was concentrated under reduced pressure at 60°C until no liquid flowed out to obtain a concentrate 1.Adding 400 mL of ethanol to the concentrate 1 and stirring it well, cooling down: Cool down to 5° C. within 10 hours to obtain a turbid liquid.The filter cake was dried to obtain 88 g of pyridoxal hydrochloride;
References:
CN107652323,2018,A Location in patent:Paragraph 0023; 0024
66-72-8
44 suppliers
inquiry

65-22-5
371 suppliers
$9.00/1g
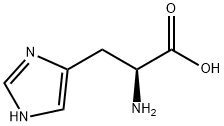
![L-Histidine, N-[[3-hydroxy-5-(hydroxymethyl)-2-methyl-4-pyridinyl]methylene]-](/CAS/20210305/GIF/14029-59-5.gif)