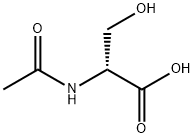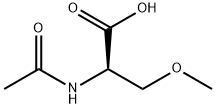
(R)-2-Acetylamino-3-methoxy-propionic acid synthesis
- Product Name:(R)-2-Acetylamino-3-methoxy-propionic acid
- CAS Number:196601-67-9
- Molecular formula:C6H11NO4
- Molecular Weight:161.16

105591-02-4
0 suppliers
inquiry

196601-67-9
26 suppliers
inquiry
Yield:196601-67-9 99.8%
Reaction Conditions:
with hydrogen;(-)-1,2-bis((2S,5S)-2,5-diphenylphospholano)ethane(1,5-cyclooctadiene)rhodium (I) tetrafluoroborate in methanol at 45; under 7500.75 Torr; for 20 h;Inert atmosphere;Sealed vessel;
Steps:
3a
Example 3a-By catalytic asymmetric hydrogenation of compounf of formula (D1)Catalyst : (S.S)-Ph-BPE-Rh = (-)-1 ,2-Bis((2S,5S)-2,5- diphenylphospholano)ethane(1 ,5-cyclooctadiene)rhodium (I) tetrafluoroborate.Hydrogenation was carried out in a 10 Vessel Multicell Reactor (Baskerville). Substrate, catalysts and solvent were charged under a nitrogen atmosphere. The substrate Z-2-Acetamido-3-methoxyacryic acid (D1) (1 g) was placed in a glass reactor which with a magnetic stirrer bar was then placed in the stainless steel high pressure vessel. Catalyst (1 mg, 0.1 %w/w) and methanol (5 mL) were added. The vessel was sealed and purged with hydrogen by pressurising the vessel to 10 barg and then releasing the pressure (2 times). Finally, the hydrogen pressure was adjusted to 10 barg and the reaction mixture was stirred at 45°C for 20 hours. The reaction was stopped by purging the vessel with helium and the reaction mixture was analysed by chemical & chiral HPLC analysis.Conversion measured by chemical HPLC showed 100% conversion of (D1) with 98.6% chemical purity. Chiral HPLC showed that 99.8% of N-acetyl-o-methyl-D-serine (C1) was obtained, hence an enatiomeric excess of 99.6%.
References:
WO2012/41986,2012,A1 Location in patent:Page/Page column 27-28

105500-34-3
0 suppliers
inquiry

196601-67-9
26 suppliers
inquiry
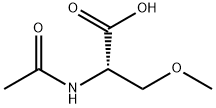
98632-99-6
28 suppliers
inquiry

196601-67-9
26 suppliers
inquiry

108-24-7
0 suppliers
$14.00/250ML
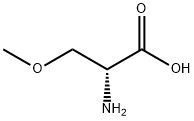
86118-11-8
128 suppliers
$8.00/100mg

196601-67-9
26 suppliers
inquiry