Rofecoxib synthesis
- Product Name:Rofecoxib
- CAS Number:162011-90-7
- Molecular formula:C17H14O4S
- Molecular Weight:314.36
103-82-2
0 suppliers
$22.19/5g
![2-Bromo-1-[4-(methylsulfonyl)phenyl]-1-ethanone](/CAS/GIF/50413-24-6.gif)
50413-24-6
188 suppliers
$19.00/5g

162011-90-7
291 suppliers
$11.00/25mg
Yield:162011-90-7 78%
Reaction Conditions:
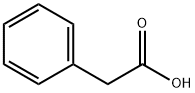
Stage #1:phenylacetic acid with sodium hydroxide in DMF (N,N-dimethyl-formamide);water at 4; for 1 h;
Stage #2:2-bromo-1-(4-methanesulfonylphenyl)ethanone with diisopropylamine at 45; for 3.5 h;
Steps:
PREPARATION OF Compound 1
A 500 mL, baffled, 3-neck round bottom flask equipped with a mechanical stirrer, temperature probe, and nitrogen inlet was charged with phenylacetic acid and DMF (150 mL). The reaction vessel was flushed with N2. To the solution was added 50 wt% NaOH, resulting in a biphasic mixture. The resulting mixture was stirred vigorously at 4 °C for one hour. The bromoketone 2 was added to the sodium phenylacetate solution. The reaction flask was protected from the light due to the known light sensitivity of Compound 1. Diisopropylamine (DIA) was added via syringe (no exotherm), and the batch was aged at 45°C for 3.5 hours. The reaction solution was cooled to 20-25°C, and 2N HCl was added over 1 h via addition funnel, maintaining the temperature between 20 and 30°C. The product was further precipitated by addition of water (32 mL, via addition funnel) to the reaction slurry over 1 hour. After ageing 1-2 hours at 25°C, the batch was filtered. The mother liquors were recycled to remove all of the product from the flask. The wet cake was washed once with 10 mL of 1:3 DMF:IPA and once with 20 mL IPA. The cake was dried by suction, to give 7.36 g of semi-pure Compound 1 (78%).
References:
MERCK & CO., INC. EP912537, 2004, B1 Location in patent:Page 13
162012-30-8
1 suppliers
inquiry

162011-90-7
291 suppliers
$11.00/25mg

201737-94-2
3 suppliers
inquiry

162011-90-7
291 suppliers
$11.00/25mg

103-82-2
0 suppliers
$22.19/5g
105-36-2
340 suppliers
$5.00/5 g

162011-90-7
291 suppliers
$11.00/25mg

103-82-2
0 suppliers
$22.19/5g
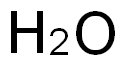
7732-18-5
506 suppliers
$12.69/100ml
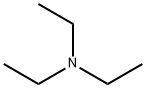
121-44-8
899 suppliers
$9.00/5g
![2-Bromo-1-[4-(methylsulfonyl)phenyl]-1-ethanone](/CAS/GIF/50413-24-6.gif)
50413-24-6
188 suppliers
$19.00/5g

162011-90-7
291 suppliers
$11.00/25mg