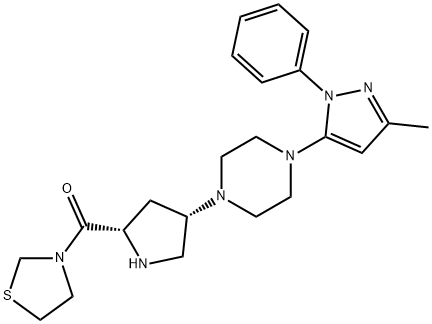
Teneligliptin synthesis
- Product Name:Teneligliptin
- CAS Number:760937-92-6
- Molecular formula:C22H30N6OS
- Molecular Weight:426.58
![(2S,4S)-4-[4-(3-Methyl-1-phenyl-1H-pyrazol-5-yl)-1-piperazinyl]-2-(3-thiazolidinylcarbonyl)-1-pyrrolidinecarboxylic acid tert-butyl ester](/CAS/20150408/GIF/401566-80-1.gif)
401566-80-1
45 suppliers
inquiry

760937-92-6
171 suppliers
$45.00/10mg
Yield:760937-92-6 93%
Reaction Conditions:
Stage #1:tert-butyl (2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]-2-(1,3-thiazolidin-3-ylcarbonyl)pyrrolidine-1-carboxylate with trifluoroacetic acid in dichloromethane at 20; for 19 h;
Stage #2: with sodium hydrogencarbonate in water
Steps:
1 3-{(2S,4S)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl}thiazolidine
3-{(2S,4S)-1-(1,1-Dimethylethyloxycarbonyl)-4-[4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl]pyrrolidin-2-ylcarbonyl}thiazolidine (25.45 g, synthesized according to the compound described in Example 222 of WO02/14271) was dissolved in dichloromethane (200 mL). Trifluoroacetic acid (50 mL) was added at room temperature, and the mixture was stirred for 19 hrs. The reaction mixture was concentrated under reduced pressure and saturated aqueous sodium hydrogencarbonate solution was added to the residue. The mixture was extracted with chloroform. The extract was washed with saturated brine and dried. The solvent was evaporated under reduced pressure. The residue was purified by silica gel column chromatography to give the title compound as a solid (19.28 g, yield 93%).
References:
Mitsubishi Pharma Corporation EP1854795, 2007, A1 Location in patent:Page/Page column 8
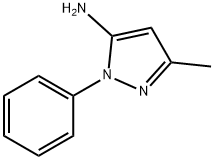
1131-18-6
252 suppliers
$10.00/25g

760937-92-6
171 suppliers
$45.00/10mg
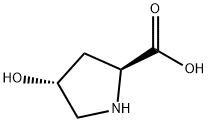
51-35-4
881 suppliers
$6.00/5g

760937-92-6
171 suppliers
$45.00/10mg
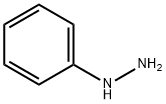
100-63-0
374 suppliers
$10.00/1g

760937-92-6
171 suppliers
$45.00/10mg
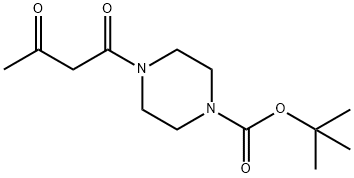
401566-77-6
23 suppliers
inquiry

760937-92-6
171 suppliers
$45.00/10mg