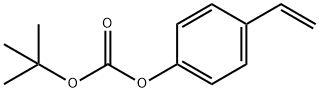
Tert-Butyl 4-Vinylphenyl Carbonate synthesis
- Product Name:Tert-Butyl 4-Vinylphenyl Carbonate
- CAS Number:87188-51-0
- Molecular formula:C13H16O3
- Molecular Weight:220.26

24424-99-5
871 suppliers
$13.50/25G
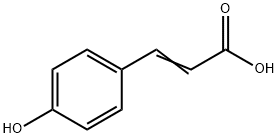
7400-08-0
459 suppliers
$29.00/10g

87188-51-0
65 suppliers
inquiry
Yield:87188-51-0 75%
Reaction Conditions:
Stage #1: para-coumaric acidwith 4-methoxy-phenol in water;N,N-dimethyl-formamide; for 6 h;Reflux;
Stage #2: di-tert-butyl dicarbonatewith dmap;triethylamine in water;N,N-dimethyl-formamide;toluene at 40; for 6 h;
Steps:
2 Synthesis of p- (tert-butoxycarbonyloxy) styrene
mixer,thermometer,A 200 ml flask equipped with a reflux condenser was charged with p-hydroxycinnamic acid 13. 12 g (80 mmol),As a polymerization inhibitor, 0.50 g of p-methoxyphenol (4 mMol),And 73.53 g of N, N-dimethylformamide as a solvent, 2.27 g of water(Moisture content of solvent: 3.0%) were charged,Under stirring,Immersed in an oil bath temperature controlled at 150 ° CHeat.Reflux was observed with increasing temperature,The temperature of the reaction solution reached 136 ° C.Heating for 6 hoursAfter continuing,Quickly cool down,45.40 g of toluene was added,60 ° C water bathWith heating,Water was azeotropically removed under a pressure of 40 Torr.Triethylamine 8.09G (80 mmol), 0.49 g (4 mmol) of N, N-dimethylaminopyridine were added, Under stirring,43.64 g (200 mmol) of di-tert-butyl dicarbonate and toluene 21.Was added dropwise at 40 ° C. over 1 hour.Subsequently, the mixture was stirred at 40 ° C. for 5 hours.The reaction solution was diluted with 20 g of toluene,After adding 80 g of water and thoroughly stirring,Minute water layer and oil layerRelease,The solvent was distilled off from the oil layer under reduced pressure to obtain a brown liquid.The obtained liquid was heated to 150 ° C.Heating with an oil bath,A simple distillation was carried out under a pressure of 2 Torr,From 109 ° C to 111 ° C13.28 g of a pale yellow transparent liquid was obtained by fractionation (75% of the theoretical yield). When chemical purity was analyzed by the above-mentioned method using HPLC,The chemical purity was 96.9% (area percentage). Also,Hydrogen nuclear magnetic resonance (1 H-NMR) spectrum was measured,The following results were obtained, so that the obtained pale yellow transparent liquid is p- (tert-butoxycarbonyloxylSi) styrene.
References:
JP2015/3890,2015,A Location in patent:Paragraph 0071
2628-16-2
405 suppliers
$7.00/1g

24424-99-5
871 suppliers
$13.50/25G

87188-51-0
65 suppliers
inquiry

24424-99-5
871 suppliers
$13.50/25G
2628-17-3
265 suppliers
$6.00/1g

87188-51-0
65 suppliers
inquiry

2628-16-2
405 suppliers
$7.00/1g

24424-99-5
871 suppliers
$13.50/25G
32503-27-8
670 suppliers
$5.00/25g

87188-51-0
65 suppliers
inquiry