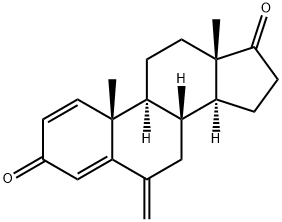
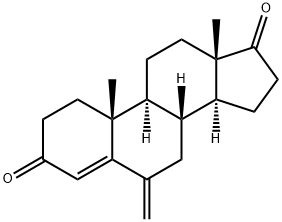
Exemestane
- CAS No.
- 107868-30-4
- Chemical Name:
- Exemestane
- Synonyms
- AROMASIN;EXALAMIDE;Exemestan;ExeMestane(AroMasin);EXE;ROMASIN;PNU155971;FCE-24304;ExeMestine;EXEMESTANE
- CBNumber:
- CB5760314
- Molecular Formula:
- C20H24O2
- Molecular Weight:
- 296.4
- MDL Number:
- MFCD00866994
- MOL File:
- 107868-30-4.mol
| Melting point | 155.13°C |
|---|---|
| Boiling point | 453.7±45.0 °C(Predicted) |
| Density | 1.13±0.1 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: ≥20mg/mL |
| form | powder |
| color | white to off-white |
| optical activity | [α]/D +250 to +300°, c = 1 in methanol |
| InChI | InChI=1S/C20H24O2/c1-12-10-14-15-4-5-18(22)20(15,3)9-7-16(14)19(2)8-6-13(21)11-17(12)19/h6,8,11,14-16H,1,4-5,7,9-10H2,2-3H3/t14-,15-,16-,19+,20-/m0/s1 |
| InChIKey | BFYIZQONLCFLEV-DAELLWKTSA-N |
| SMILES | C1(=O)C=C2[C@](C)(C=C1)[C@]1([H])[C@]([H])([C@@]3([H])[C@@](CC1)(C)C(=O)CC3)CC2=C |
| CAS DataBase Reference | 107868-30-4(CAS DataBase Reference) |
| NCI Dictionary of Cancer Terms | Aromasin; exemestane |
| FDA UNII | NY22HMQ4BX |
| NCI Drug Dictionary | Aromasin |
| ATC code | L02BG06 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |   GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H360-H411 | |||||||||
| Precautionary statements | P201-P202-P273-P280-P308+P313-P391 | |||||||||
| Hazard Codes | T,N | |||||||||
| Risk Statements | 60-61-51 | |||||||||
| Safety Statements | 53-22-36/37-57 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29372900 | |||||||||
| NFPA 704 |
|
Exemestane price More Price(29)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | PHR1634 | Exemestane Pharmaceutical Secondary Standard; Certified Reference Material | 107868-30-4 | 1g | $128 | 2024-03-01 | Buy |
| Sigma-Aldrich | 1269050 | Exemestane United States Pharmacopeia (USP) Reference Standard | 107868-30-4 | 150mg | $436 | 2024-03-01 | Buy |
| Cayman Chemical | 15008 | Exemestane ≥95% | 107868-30-4 | 5mg | $44 | 2024-03-01 | Buy |
| Cayman Chemical | 15008 | Exemestane ≥95% | 107868-30-4 | 10mg | $70 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0001756 | Exemestane for system suitability European Pharmacopoeia (EP) Reference Standard | 107868-30-4 | y0001756 | $220 | 2024-03-01 | Buy |
Exemestane Chemical Properties,Uses,Production
Indications and Usage
Exemestane is an irreversible steroid aromatase inhibitor. Its structure is similar to that of aromatase’s natural substrate, androstenedione, and acts as a pseudosubstrate. Postmenopausal women’s estrogen is mainly converted from androgen (produced by the adrenal cortex) by aromatase in the surrounding tissue. This drug irreversibly binds with the active site on aromatase to deactivate it, thus dramatically lower estrogen levels in the blood circulation of postmenopausal women. By inhibiting aromatase to lower estrogen levels, it can be used to treat hormone-dependent breast cancer in postmenopausal women.
Exemestane is suitable for treating advanced breast cancer in naturally or artificially postmenopausal women that has not responded well to tamoxifen treatment. It is also suitable for treating estrogen and progesterone receptor positive postmenopausal advanced breast cancer, and it can also be used to treat metastasized breast cancer and as adjuvant therapy for early breast cancer.
Pharmacokinetics
This drug has no noticeable effect on adrenal corticosteroids biosynthesis. Even when its concentration is over 600 times the concentration required to inhibit aromatase, it still has no noticeable effect the other enzymes in the corticosteroid production pathway.
This drug is absorbed quickly when taken orally and will affect food absorption. Its oral bioavailability is 42%. Postmenopausal women have a higher absorption rate than healthy test subjects. Patients reach peak blood concentration 2-4 hours after intake, and the peak lasts for an average of 1.2 hours, which is 2.9 hours shorter than healthy subjects. It mainly binds to Α1-acid glycoprotein and protein, and its overall binding rate to protein is 90%. It is mainly metabolized by the liver, the metabolite is inactive 17-Hydrecoxetron, and its clearing half-life is 24 hours. It is mostly excreted through urine and feces, which both account for 42% of the consumed amount.
Drug interactions
1. This drug cannot be used in combination with estrogen-based drugs to avoid counteracting its medicinal effects.
2. Exemestane is mostly metabolized by CYP3A4, but when used in combination with a strong CYP3A4 inhibitor (Ketoconazole), its pharmacokinetics do not exhibit any change. This is because the inhibitor does not seem to affect the drug’s pharmacokinetics, but it is also possible that the known CYP3A4 inducer lowers the blood concentration of Exemestane.
Side effects
Adverse effects include nausea, dryness, constipation, diarrhea, dizziness, insomnia, rash, fatigue, fever, swelling, pain, vomiting, abdominal pain, increased appetite, weight gain, etc. Additionally, some literature reports cases of hypertension, depression, anxiety, difficulty breathing, coughing, etc. There may also be decreases lymph cell amounts, abnormalities in liver function indicators (such as alanine aminotransferase), etc.
Clinical Research
For patients resistant to tamoxifen, 25mg of Exemestane, qd, can achieve an objective efficacy rate of 15-28% and a median continuation period of 69-76 weeks. Exemestane is superior to Megestrol, and it can extend the disease progression time. For patients who have worsened conditions following Megestrol treatment, Exemestane can still achieve an objective efficacy rate of 11-13%. A daily 25mg dose of Exemestane has an objective efficacy rate of 6.6% on patients who have not responded to non-steroidal aromatase inhibitors, and the two drugs are not cross-resistant.
Contradictions
1. Not to be used by patients allergic to this drug.
2. Not to be used by pregnant women, breastfeeding women, and children.
Warnings and precautions
1. Premenopausal women usually do not use Exemestane.
2. Patients with moderate to severe liver or renal failure should use with caution. Exceeding the recommended dosage of Exemestane may increase the occurrence of nonfatal adverse effects.
3. FDA labeled this drug’s pregnancy safety as level D.
4. Elderly patients do not require any special precautions.
Description
Exemestane was launched in US and other countries for the treatment of estrogendependent tumors and postmenopausal breast cancer. It is a novel steroidal compound structurally related to the natural substrate for the biosynthesis of estrogen, androstanedione, and can be synthesized by methylidenation of androsta-1, 4-dien- 17beta-ol-3-one in 6 position then oxidation of the alcohol function. Exemestane is an irreversible inactivator of the aromatase enzyme system, so inducing in vivo a dose-related sustained suppression of serum estrogen and minimal endocrine activity. It is the first steroidal representative of the third-generation of orally active aromatase inhibitors with a highly potent and selective mechanism of action, displaying good tolerability and safety profile. In rats with DMBA-induced mammary tumors, 10 to 100 mglkg of exemestan administered po twice-daily for 4 weeks resulted in 76 to 88% regression. In women failing anti-estrogen therapy with tamoxifen, this agent has demonstrated high activity in locally advanced or metastatic disease. In addition, it may also have potential for breast cancer prevention.
Chemical Properties
white to light yellow crystal powder
Originator
Farmitalia Carlo Erba (Italy)
Uses
antiinfective
Uses
An antineoplastic (hormonal)
Uses
Labeled Exemestane, intended for use as an internal standard for the quantification of Exemestane by GC- or LC-mass spectrometry.
Definition
ChEBI: A 17-oxo steroid that is androsta-1,4-diene-3,17-dione in which the hydrogens at position 6 are replaced by a double bond to a methylene group. A selective inhibitor of the aromatase (oestrogen synthase) system, it is used in the treatment of advanced brea t cancer.
Manufacturing Process
0.50 g of 6-methylenandrost-4-ene-3,17-dione and 0.57 g of dichlorodicyanobenzoquinone were refluxed in 20 ml of anhydrous dioxane for about 15 hours. To remove the DDQ the suspension was filtered through alumina. After evaporation of the solvent the residue was dissolved in ethyl acetate, the organic layer washed with water, dried over sodium sulfate and the solvent removed under vacuum. The crude product was chromatographed on silica gel using hexane/ethyl acetate to yield 0.25 g of pure 6- methylenandrosta-1,4-diene-3,17-dione, m.p. 188-191°C, λmax 247 nm (ε 13.750).
brand name
Aromasin (Pharmacia& Upjohn).
Therapeutic Function
Antineoplastic
General Description
Exemestane, 6-methylenandrosta-1,4-diene-3,17-dione (Aromasin), is the first steroid-basedaromatase inhibitor approved for the treatment of breastcancer in the United States. It is a mechanism-based inactivatorthat irreversibly inhibits the enzyme. Plasma estrogenlevels are reduced by 85% to 95% within 2 to 3 days, and effectslast 4 to 5 days. Exemestane does not inhibit any of themajor cytochromes P450 and has essentially no interactionwith steroid receptors, with only a very weak affinity for theAR. The 17β-hydroxyexemestane reduction product, however,has much higher affinity for the AR than the parent(still several fold less than DHT, 0.28% for parent vs. 30%for metabolite). The clinical significance of the affinity islikely minimal because of the low levels of the metaboliteproduced.
Hazard
A reproductive hazard.
Biochem/physiol Actions
Exemestane is a steroidal antiestrogen and irreversible aromatase inhibitor. Exemestane acts as a false substrate for the aromatase enzyme. Exemestane also prevents the conversion of androgens to estrogens and is used to treat estrogen-dependent breast cancer.
Clinical Use
Irreversible, steroidal aromatase inhibitor:Treatment of breast cancer
Metabolism
Metabolised via oxidation by the cytochrome P450 isoenzyme CYP3A4, and via reduction by aldoketoreductase. Metabolites are excreted in the urine (39-45%) and faeces (36-48%).
References
[1] franco buzzetti, enrico di salle, antonio longo, gabriella briatico. synthesis and aromatase inhibition by potential metabolites of exemestane (6-methylenandrosta-1,4-diene-3,17-dione). steroids. november 1993. 58(11): 527-532.
[2] gustavo de albuquerque cavalcanti, bruno carius garrido, felipe dias leal, monica costa padilha, xavier de la torre, francisco radler de aquino neto. detection of new urinary exemestane metabolites by gas chromatography coupled to mass spectrometry. steroids. september–october 2011. 76(10-11): 1010-1015.
[3] stephanie a. jones, stephen e. jones. exemestane: a novel aromatase inactivator for breast cancer. clinical breast cancer. october 2000. 1(3): 211-216.
Exemestane Preparation Products And Raw materials
Raw materials
1of4
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Shanghai Longyu Biotechnology Co., Ltd. | +8615821988213 | info@longyupharma.com | China | 2531 | 58 |
| Changzhou Xuanming Pharmaceutical Technology Co., Ltd. | +8618068519287 | sales@xuanmingchem.com | China | 822 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10523 | 58 |
| qingdao future trading Co., Ltd | +86-13335044410 +86-13335044410 | cui56813@163.com | China | 110 | 58 |
| Hong Kong Excellence Biotechnology Co., Ltd. | +86-86-18838029171 +8618126314766 | ada@sh-teruiop.com | China | 893 | 58 |
| Shanghai Getian Industrial Co., LTD | +86-15373193816 +86-15373193816 | mike@ge-tian.com | China | 272 | 58 |
| Guangdong Tuoyuan Biotechnology Co., LTD | +85267545345 | sterodschina@gmail.com | China | 129 | 58 |
| CONTIDE BIOTECH CO.,LTD | +85253358525 | xena@healthtide-api.com | China | 499 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12456 | 58 |
| Hebei Yanxi Chemical Co., Ltd. | +8617531190177 | peter@yan-xi.com | China | 5993 | 58 |
Related articles
- Exemestane: A Breakthrough Aromatase Inhibitor Treatment for Advanced Breast Cancer
- Exemestane effectively treats hormone-sensitive breast cancer in postmenopausal women, showing superior efficacy and safety in....
- Feb 7,2024
- Exemestane used to treat breast cancer
- Exemestane is an oral steroidal aromatase inhibitor used to treat hormone responsive breast cancers in women who are postmenop....
- Jan 11,2023
- The Applications and Pharmacological effects of Exemestane
- Exemestane is the generic name for the trade name drug Aromasin?. In some cases, health care professionals may use the trade n....
- Aug 20,2019
View Lastest Price from Exemestane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-04-26 | ExeMestane(AroMasin)
107868-30-4
|
US $9.90-3489.00 / g | 1g | 99.99% | 2 tons | Wuhan Cell Pharmaceutical Co., Ltd | |
 |
2024-04-26 | Exemestane
107868-30-4
|
US $9.90-3489.00 / g | 1g | 99% | 100kg | Wuhan Cell Pharmaceutical Co., Ltd | |
 |
2024-04-26 | Exemestane
107868-30-4
|
US $20.00-10.00 / kg | 1kg | 98% | 20 | Hebei Kangcang new material Technology Co., LTD |
-

- ExeMestane(AroMasin)
107868-30-4
- US $9.90-3489.00 / g
- 99.99%
- Wuhan Cell Pharmaceutical Co., Ltd
-

- Exemestane
107868-30-4
- US $9.90-3489.00 / g
- 99%
- Wuhan Cell Pharmaceutical Co., Ltd
-

- Exemestane
107868-30-4
- US $20.00-10.00 / kg
- 98%
- Hebei Kangcang new material Technology Co., LTD