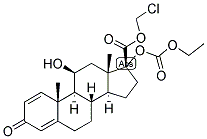
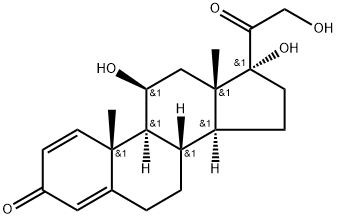
Loteprednol etabonate
- CAS No.
- 82034-46-6
- Chemical Name:
- Loteprednol etabonate
- Synonyms
- Etabonate;Alrex;HGP 1;P 5604;Lotemax;CDDD 5604;Etocloteprednol;LOTEPREDNOL ETABONATE;Lotoprednol etabonate;Loteprednol Impurity 10
- CBNumber:
- CB9396463
- Molecular Formula:
- C24H31ClO7
- Molecular Weight:
- 466.95
- MDL Number:
- MFCD00870765
- MOL File:
- 82034-46-6.mol
| Melting point | 220.5-223.5 |
|---|---|
| Boiling point | 600.1±55.0 °C(Predicted) |
| Density | 1.31±0.1 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: soluble5mg/mL, clear (warmed) |
| form | powder |
| pka | 14.06±0.70(Predicted) |
| color | white to beige |
| Water Solubility | <1mg/L(23 ºC) |
| Stability | Hygroscopic |
| InChIKey | DMKSVUSAATWOCU-ICASLIHPSA-N |
| FDA UNII | YEH1EZ96K6 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H361-H413 | |||||||||
| Precautionary statements | P501-P273-P202-P201-P280-P308+P313-P405 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 2937.22.0000 | |||||||||
| NFPA 704 |
|
Loteprednol etabonate price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | SML0547 | Loteprednol Etabonate ≥98% (HPLC) | 82034-46-6 | 10mg | $99.3 | 2024-03-01 | Buy |
| Sigma-Aldrich | SML0547 | Loteprednol Etabonate ≥98% (HPLC) | 82034-46-6 | 50mg | $241.2 | 2024-03-01 | Buy |
| TCI Chemical | L0327 | Loteprednol Etabonate | 82034-46-6 | 50MG | $112 | 2024-03-01 | Buy |
| TCI Chemical | L0327 | Loteprednol Etabonate | 82034-46-6 | 250MG | $385 | 2024-03-01 | Buy |
| Cayman Chemical | 23305 | Loteprednol etabonate ≥98% | 82034-46-6 | 5mg | $37 | 2024-03-01 | Buy |
Loteprednol etabonate Chemical Properties,Uses,Production
Description
Loteprednol (as Loteprednol Etabonate) is a topical anti-inflammatory corticosteroid. Loteprednol etabonate (LE) has a 17α-chloromethyl ester, in lieu of a ketone group, and a 17β-etabonate group. LE is highly lipophilic and binds with high affinity to the glucocorticoid receptor. Any unbound LE is metabolized to inactive metabolites.
Loteprednol etabonate is used in ophthalmic solution for the treatment of steroid responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the globe such as allergic conjunctivitis, uveitis, acne rosacea, superficial punctate keratitis, herpes zoster keratitis, iritis, cyclitis, and selected infective conjunctivitis. It is used in ophthalmic ointment for the treatment of post-operative inflammation and pain following ocular surgery. As a nasal spray, it is used for the treatment and management of seasonal allergic rhinitis.
References
[1] http://www.webmd.com
[2] https://www.drugbank.ca
[3] http://www.bausch.com
[4] Timothy L. Comstock, Heleen H. DeCory (2012) Advances in Corticosteroid Therapy for Ocular Inflammation: Loteprednol Etabonate, International Journal of Inflammation, 2012, 789623
[5] N. Krug, JM. Hohlfeld, H. Geldmacher, M Larbig, R. Heermann, N. Lavallee, DT. Nguyen, U. Petzold, R. Hermann (2005) Effect of loteprednol etabonate nasal spray suspension on seasonal allergic rhinitis assessed by allergen challenge in an environmental exposure unit, Allergy, 60, 354-359
Description
Loteprednol etabonate was introduced in the US as Lotemax (opththalmic suspension at 0.5%) for the treatment of steroid-responsive inflammatory conditions of the palpebral and bulbar conjunctiva, cornea and anterior segment of the ocular globe, and as Alrex (opththalmic suspension at 0.2%) for the symptomatic treatment of seasonal allergic conjunctivitis. Loteprednol etabonate is a novel soft corticosteroid with a superior efficacy and an improved safety profile compared to prior ophthalmic steroids due to its metabolic lability and a fast enzymatic transformation to inactive metabolite. A combination of Lotemax with the antibiotic Tobramycin is currently under development.
Chemical Properties
x
Originator
Pharmos (US)
Uses
An ophthalmic corticosteroid. Used as an anti-inflammatory.
Uses
Biological Activity Chemical Information Tech Support & FAQs Biological Activity Loteprednol etabonate is an anti-inflammatory corticosteroid used in ophthalmology. It is used for the treatment of steroid responsive inflammatory conditions of the eye su
Definition
ChEBI: Loteprednol etabonate is an etabonate ester, an 11beta-hydroxy steroid, a steroid ester, an organochlorine compound, a steroid acid ester and a 3-oxo-Delta(1),Delta(4)-steroid. It has a role as an anti-inflammatory drug. It is functionally related to a loteprednol.
Manufacturing Process
To a solution of hydrocortisone (15 g, 0.04 mol) in 120 ml of THF and 30 ml of methanol at room temperature is added a warm solution of sodium metaperiodate (25.7 g, 0.12 mol) in 100 ml of water. The reaction mixture is stirred at room temperature for 2 hours, then is concentrated under reduced pressure to remove the tetrahydrofuran and methanol. The solid is triturated with 50 ml of water, separated by filtration, washed with water and dried in vacuo at 50°C for 3 hours. The product, 11β,17α-dihydroxyandrost-4-en-3- one-17β-carboxylic acid (i.e., cortienic acid), is obtained in approximately 96% yield (13.76 g); melting point 231-234°C.
To a cold solution of 11β,17α-dihydroxyandrost-4-en-3-one-17β-carboxylic acid
(5% weight/volume; 1 mol) and triethylamine (4 mol) in dichloromethane is
added a 50% (weight/volume) solution of ethyl chloroformate (3.9 mol) in
dichloromethane. The reaction mixture is allowed to warm to room
temperature over a 2 hour period. The triethylamine hydrochloride precipitate
which forms is removed by filtration and the filtration is washed successively
with 3% sodium bicarbonate, 1% hydrochloric acid and water. The organic
layer is separated, dried with magnesium sulfate, and filtered. The filtrate is
concentrated in vacuo to a foam.
The foam is used in the next step below or chromatographed and crystallized
for analysis. The product 17α-ethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-
one-17β-carboxylic acid, melting at 192-195°C C after chromatography and
crystallization.
17α-Ethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-one-17β-carboxylic acid is
combined with an equivalent amount of 1 N sodium hydroxide in methanol
and that solution is diluted to 100 times the original volume with ethyl ether.
The suspension which results is refrigerated for 1 hour. Then, the crystals
which form are removed by filtration, dried in an evacuated desiccator, and
dissolved in hexamethylphosphoramide (10% weight/volume). A portion of the
resultant solution containing 1 mole of the acid salt, i.e. of sodium 17αethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-one-17β-carboxylate, is
combined with 4 moles of chloromethyl iodide. The reaction mixture is
maintained at room temperature for 3 hours, then is diluted to 10 times the
original volume with ethyl acetate. The diluted reaction mixture is washed
successively with 5% sodium thiosulfate, 3% sodium bicarbonate, and water.
The organic layer is separated, dried with magnesium sulfate and filtered. The
filtrate is concentrated in vacuo to a foam. The foam is purified by
crystallization from ethyl ether or tetrahydrofuran/hexane. There is thus
obtained chloromethyl-17α-ethoxycarbonyloxy-11β-hydroxyandrost-4-en-3-
one-17β-carboxylate, melting at 197-200°C after crystallization.
brand name
Alrex (Bausch & Lomb); Lotemax (Bausch & Lomb); Lotemax (Pharmos);Lotemax (0.5%).
Therapeutic Function
Glucocorticoid
General Description
Loteprednol etabonate,chloromethyl 17α-[(ethoxycarbonyl)oxy]-11β-hydroxy-3-oxoandrosta-1,4-diene-17-carboxylate (Alrex,Lotemax), has a modified carboxylate at the C17 positionrather than the typical ketone functionality. This modificationmaintains affinity for the GR but allows facile metabolismto inactive metabolites. This limits the systemic actionof the drug. Loteprednol etabonate is used as anophthalmic suspension that has greatly reduced systemicaction because of rapid metabolism to the inactive carboxylate.
Biochem/physiol Actions
Loteprednol Etabonate is an anti-inflammatory corticosteroid (ophthalmology).
Loteprednol etabonate Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Shanghai Yingrui Biopharma Co., Ltd. | +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 |
| Jinan Chenghui-Shuangda Chemical Co.,Ltd | +86-531-58897082 | ericqiao@jnchsd.com | CHINA | 158 | 58 |
| Jinan Jianfeng Chemical Co., Ltd | 0531-88110457; +8615562555968 | info@pharmachemm.com | China | 222 | 58 |
| Jinan Shengqi pharmaceutical Co,Ltd | 86+18663751872 | christine@shengqipharm.com | CHINA | 491 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| HubeiwidelychemicaltechnologyCo.,Ltd | 18627774460 | faith@widelychemical.com | CHINA | 742 | 58 |
| Standardpharm Co. Ltd. | 86-714-3992388 | overseasales1@yongstandards.com | United States | 14336 | 58 |
| BOC Sciences | +1-631-485-4226 | inquiry@bocsci.com | United States | 19553 | 58 |
View Lastest Price from Loteprednol etabonate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2021-07-13 | Loteprednol Etabonate
82034-46-6
|
US $15.00-10.00 / KG | 1KG | 99%+ HPLC | Monthly supply of 1 ton | Zhuozhou Wenxi import and Export Co., Ltd | |
 |
2021-05-11 | Loteprednol etabonate
82034-46-6
|
US $100.00 / KG | 1KG | 99% | 1000t | Hebei Minshang Biological Technology Co. LTD | |
 |
2020-09-02 | Loteprednol etabonate
82034-46-6
|
US $150.00 / KG | 1KG | 99.9% | 10tons | Hebei Runbin Biotechnology Co. LTD |
-

- Loteprednol Etabonate
82034-46-6
- US $15.00-10.00 / KG
- 99%+ HPLC
- Zhuozhou Wenxi import and Export Co., Ltd
-

- Loteprednol etabonate
82034-46-6
- US $100.00 / KG
- 99%
- Hebei Minshang Biological Technology Co. LTD
-

- Loteprednol etabonate
82034-46-6
- US $150.00 / KG
- 99.9%
- Hebei Runbin Biotechnology Co. LTD