Thiophenes compound is a five-membered heterocyclic compound containing only one sulfur atom with the basic structure being thiophene. Thiophene is presented in the shale oil and coal tar.
Thiophene is more active than benzene; it has photosensitivity with light shining causing carbon skeleton rearrangement; it can react with the metal; the sulfur in thiophene ring, under the action of the Ramey nickle, can be removed; substitution reaction can occur (mostly in the position 2); it can have ring open reaction upon being oxidized with different oxidizing agents being able to give different products; it can have nitration in the 2,5 position, generating nitro thiophene; it can be subject to halogenation, generating position 2 single halide, 2, 5-dihalide, 2, 3, 5-trihalides and tetrahalides; it can also have condensation reaction with benzene, generating benzothiophene.
Thiophene ring, due to that the unshared electron pair of the sulfur can form two carbon - carbon together into 6π electron system, with aromatic properties. Therefore, it can be subject to nitrification, sulfonation, halogenation and other electrophilic substitution reactions. The substituted positions include 2-position and 5-positions. It is much easier for thiophene to have electrophilic substitution reaction than benzene. E.g., thiophene, in the presence of mild condensation agent such as orthophosphoric acid can have Friedel-Crafts reaction with acyl group being introduced to the alpha- position.
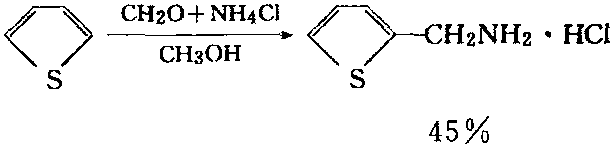
In the presence of nitric acid, the thiophene can have reaction with iodide to generate 2-iodo-thiophene with a higher yield. Alternatively, you can also easily apply Mannich reaction to prepare 2-thienyl methylamine.
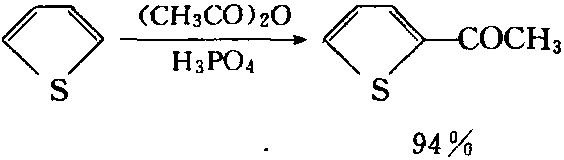
Thiophene can be used in the production of various kinds of dyes, perfumes, thermal shock resistant plastic, highly active solvent, stimulating hormone, insecticide, brightening agents, cosmetics and bio-activating substances and vitamins, anesthetics and antibiotics and other drugs. Thiophene, although may be chemically synthesized to make, but the cost is too high. Thiophene mainly exists in the light benzene obtained through pre-distillation of crude benzene. When the light benzene is subject to refinement through hydrogenation, the thiophene ring can be destroyed. When the light benzene undergoes pickling for refining, most of thiophene and unsaturated compounds can subject to polymerization into tar-like substance with only a small amount of sulfuric acid reacting with thiophene to generate easily extractable thiophene sulfonic acid, which greatly reduces the yield of thiophene.
When the light benzene undergoes pickling and refining, sulfuric acid can react with thiophene to generate thiophene-sulfonic acid to be dissolved in sulfuric acid. The waste sulfuric acid is clarified; the tarry substance was removed, followed by hydrolysis distillation. The distillate, after undergoing condensed cooling and separation, can give crude distilled oil containing thiophene and aromatic hydrocarbons. The crude distilled oil, after neutralization with alkaline to neutral or slightly alkaline, was further distilled in a distillation column with a theoretical plate number of 30 to 40, giving the product with thiophene content being higher than 90%. Upon distillation, the middle fraction was separated and concentrated to flow back into the crude oil distillate. After the distillation of all the thiophene, from the distillation residue, we can also obtain inter-xylene product with a purity being around 95%.