- Propylene glycol
-

- $1.00 / 1g
-
2024-04-26
- CAS:57-55-6
- Min. Order: 1g
- Purity: 99%
- Supply Ability: 1000kg
- propylene glycol
-

- $2700.00 / 1T
-
2024-04-26
- CAS:57-55-6
- Min. Order: 10KG
- Purity: 99%
- Supply Ability: 10000 kilograms/ month
Related articles - Applications of Propylene glycol
- Propylene glycol is a synthetic, colorless, odorless, tasteless liquid that belongs to the same chemical class as alcohol.
- Nov 7,2019
|
| | Propylene glycol Chemical Properties |
| Melting point | -60 °C (lit.) | | Boiling point | 187 °C (lit.) | | density | 1.036 g/mL at 25 °C (lit.) | | vapor density | 2.62 (vs air) | | vapor pressure | 0.08 mm Hg ( 20 °C) | | refractive index | n20/D 1.432(lit.) | | FEMA | 2940 | PROPYLENE GLYCOL | | Fp | 225 °F | | storage temp. | Store at +5°C to +30°C. | | solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) | | pka | 14.49±0.20(Predicted) | | form | Viscous Liquid | | Specific Gravity | 1.038 (20/20℃)1.036~1.040 | | color | APHA: ≤10 | | PH | 6-8 (100g/l, H2O, 20℃) | | Odor | at 100.00 %. odorless very slight alcoholic | | Odor Type | odorless | | explosive limit | 2.4-17.4%(V) | | Water Solubility | miscible | | Sensitive | Hygroscopic | | JECFA Number | 925 | | Merck | 14,7855 | | BRN | 1340498 | | Dielectric constant | 32.0 | | InChIKey | DNIAPMSPPWPWGF-UHFFFAOYSA-N | | LogP | -0.92 | | CAS DataBase Reference | 57-55-6(CAS DataBase Reference) | | NIST Chemistry Reference | Propylene glycol(57-55-6) | | EPA Substance Registry System | Propylene glycol (57-55-6) |
| Safety Statements | 24/25 | | WGK Germany | 1 | | RTECS | TY2000000 | | Autoignition Temperature | 779 °F | | TSCA | Yes | | HS Code | 29053200 | | Hazardous Substances Data | 57-55-6(Hazardous Substances Data) | | Toxicity | LD50 orally in Rabbit: 19400 - 36000 mg/kg LD50 dermal Rabbit 20800 mg/kg |
| | Propylene glycol Usage And Synthesis |
| Physical and Chemical Properties | Propylene glycol is scientifically named as “1,2-propanediol”, and has a chemical formula of CH3CHOHCH2OH and a molecular weight of 76.10. There is a chiral carbon atom in the molecule. Its racemate is a hygroscopic viscous liquid and is slightly spicy. It has a specific gravity of 1.036 (25/4 °C), a freezing point of-59 °C, and a boiling point of 188.2 °C, respectively 83.2 °C (1,333 Pa). It is miscible with water, acetone, ethyl acetate and chloroform, and is soluble in ether. It is soluble in many essential oils, but is not miscible with petroleum ether and paraffin oil. It is relatively stable to heat and light, and is more stable at low temperatures. Its L-isomer has a boiling point of 187 to 189 °C and a specific optical rotation [α] of D20-15.0°. It can be oxidized at high temperatures to propionaldehyde, lactic acid, pyruvic acid and acetic acid.
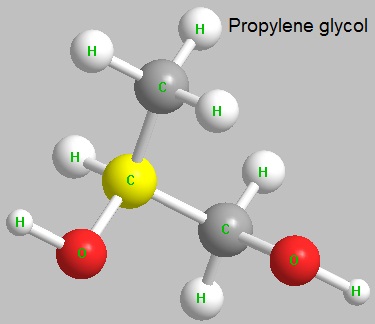
Figure 1 the molecular structure of propylene glycol.
Propylene glycol is a diol having the general nature of the alcohol. It can react with inorganic and organic acids to generate mono-or di-esters. It reacts with propylene oxide to generate ether, with hydrogen halide to generate halohydrin, and with acetaldehyde to generate methyl dioxolane.
| | Medicinal property and application | Propylene glycol has good solubility and less toxicity and irritation, and is widely used as solvents, extraction solvents and preservatives for injections (eg. intramuscular injections, intravenous injections) and non-injectable pharmaceutical preparations (such as oral liquid, ophthalmic preparations, otic preparations, dental preparations, rectovaginal preparations, transdermal preparations, etc.). It is better than glycerol solvent and can dissolve many substances such as corticosteroids (sex hormone), chloramphenicol, sulfonamides, barbiturate, reserpine, quinidine, corticosterone acetate, tetrahydropalmatine sulfate, mechlorethamine hydrochloride, vitamin A, vitamin D, many volatile oils, most of the alkaloids and many local anesthetics.
Propylene glycol is similar to ethanol when used as a bacteriostatic agent, and its efficacy to inhibit mold is similar to glycerin and is slightly lower than that of ethanol. Propylene glycol is commonly used as a plasticizer for the aqueous film coating materials. Its mixture with equal amounts of water can delay the hydrolysis of certain drugs, and increase the stability of the preparation product. It is used as an antimicrobial preservative in 15% to 30% propylene glycol solution and semi-solid formulation, as humectants in about 15% propylene glycol topical formulation, and as solvent and co-solvent in 10% to 30% propylene glycol aerosol solvent, 10% to 25 % propylene glycol oral solution, 10% to 60% injectable formulation and 5% to 80% topical formulation.
[Stability and storage condition] It is very stable at room temperature, but is oxidized when left open at high temperatures (above 280 °C); has a chemical stability after mixing with 95% ethanol or water; can be sterilized by autoclaving or sterile filtration. Propylene glycol has hygroscopicity, and should be positioned at cool and dry place and stored in dark airtight container.
[Incompatibility] It has incompatibility with some oxidants (such as potassium permanganate).
The above information is edited by the Chemicalbook of Jin Yinxue.
| | Content analysis | A 10μl sample is injected into the gas chromatograph, which has a thermal conductivity detector. The column is 1m × 6.35mm stainless steel column. The filler is polyethylene glycol 20M (Carbowax compound 20M) 4%, and the carrier is a 40/60 mesh sieved polytetrafluoroethylene (Chromosorb T) or similar material. Helium carrier gas has a flow rate of 75ml/min. Injector temperature is 240 °C; column temperature is 120 to 200 °C, temperature increment is 5 °C/min; final temperature is 250 °C. Under specified conditions, the residence time of propylene glycol is about 5.7 minutes, the residence time of three kinds of glycol isomers are respectively 8.2, 9.0 and 10.2 minutes. The area of each peak is determined using any proper method, and then the percentage of propylene glycol area is calculated and transformed into mass percentage.
| | Toxicity | FAO/WHO (2000): ADI is 0 to 25mg/kg.
LD50 is 22 to 23.9 mg/kg (mouse, oral).
GRAS (FDA, §184.1666, 2000).
| | Use limitation | FAO/WHO (1984): Cottage cheese, the cream mixture amount of 5g/kg (used alone or in combination with other carriers and stabilizers).
Japan (1998): Raw noodles, raw stuffing and cuttlefish smoked products ≤2%; skins for dumplings, steamed dumplings, spring rolls and wonton ≤1.2%; other food ≤0.6%.
GB 2760-96: pastry 3.0g/kg, chewing gum.
FDA, §184.1666 (2000): Alcoholic beverages 5%; frosting and candy 24%; frozen dairy 2.5%; flavoring agents, flavor enhancers 97%; nuts and nut products 5%; other food 2.0%.
| | Uses | Propylene glycol is used for similar applications as other glycols.
Propylene glycol is an important raw material for unsaturated polyester, epoxy resin, and polyurethane resin. The use amount in this area accounts for about 45% of the total consumption of propylene glycol. Such unsaturated polyester is used extensively for reinforced plastics and surface coatings. Propylene glycol is excellent in viscosity and hygroscopicity and is non-toxic, and thus is widely used as hygroscopic agent, antifreeze, lubricants and solvents in the food, pharmaceutical and cosmetic industry. In the food industry, propylene glycol reacts with fatty acid to give propylene ester of fatty acids, and is mainly used as food emulsifier; Propylene glycol is a good solvent for flavorings and pigments. Propylene glycol is commonly used as solvents, softeners and excipients, etc. in the pharmaceutical industry for the manufacture of various types of ointments and salves. Propylene glycol is also used as a solvent and a softener for cosmetic since it has good mutual solubility with various spices. Propylene glycol is also used as tobacco moisturizing agents, antifungal agents, food processing equipment lubricants and solvents for food marking ink. Aqueous solution of propylene glycol is an effective anti-freeze agent.
| | Synthesis method | It can be obtained by hydrolysis of Propylene oxide:
CH3CHCH2+H2O[H+]→CH3CH(OH)CH2OH
Direct hydration
Propylene oxide and water are fed in a molar ratio of 1: 15, and react at 150-2000 °C, a pressure of 1.2-1.4 MPa for 30 minutes to obtain 16% aqueous solution of propylene glycol, which is subjected to evaporation to obtain the finished product.
Catalyzed hydrolysis
The reaction is performed under catalyzation of sulfuric acid or hydrochloric acid. 0.5% to 1.0% dilute sulfuric acid is added into 10% to 15% aqueous solution of propylene oxide, the mixture is hydrolyzed at 50 to 70 °C; the hydrolysate is neutralized and concentrated under reduced pressure, and refined to obtain the finished products.
The preparation method is a method in which propylene oxide is hydrolyzed to propylene glycol, and which can be carried out in the liquid phase. There are catalytic and non-catalytic processes in industry. Catalytic method is a method in which hydrolysis is carried out in the presence of 0.5% to 1% sulfuric acid at 50 to 70 °C. Non-catalytic process is carried out under high temperature and pressure (150 to 300℃, 980 to 2940kPa), and is used for production in domestic.
| | Acute toxicity | Oral-rat LD50: 20000 mg/kg; Oral-Mouse LD50: 32000 mg/kg
| | Irritation data | Eyes-rabbit 100 mg mild
| | Extinguishing agent | Dry powder, foam, sand, water. | | Description | Propylene glycol is used as antifreeze in breweries and
diaries, in the manufacture of resins, as a solvent, and
as an emulsifier in food. It was present as an
occupational sensitizer in the color-film developer
Flexicolor. | | Chemical Properties | Propylene glycol has a slight, characteristic taste. It is practically odorless. It absorbs moisture when exposed to moist
air. | | Chemical Properties | Propylene glycol is a colorless, odorless,
syrupy liquid. | | Occurrence | Reported found in several varieties of mushrooms, roasted sesame seed, oat groats, parmesan cheese, cocoa,
pecans and truffle. | | Uses | Used as a solvent. | | Uses | Propylene Glycol is a humectant and flavor solvent that is a polyhy-
dric alcohol (polyol). it is a clear, viscous liquid with complete solu-
bility in water at 20°c and good oil solvency. it functions as a
humectant, as do glycerol and sorbitol, in maintaining the desired
moisture content and texture in foods such as shredded coconut
and icings. it functions as a solvent for flavors and colors that are
insoluble in water. it is also used in beverages and candy. | | Uses | Next to water, propylene glycol is the most common moisturecarrying vehicle used in cosmetic formulations. It has better skin permeation than glycerin, and it also gives a pleasant feel with less greasiness than glycerin. Propylene glycol is used as a humectant because it absorbs water from the air. It also serves as a solvent for anti-oxidants and preservatives. In addition, it has preservative properties against bacteria and fungi when used in concentrations of 16 percent or higher. There is a concern that propylene glycol is an irritant at high concentrations, though it appears to be quite safe at usage levels under 5 percent. | | Definition | An alcohol in which the hydroxyl groups are attached to a carbon atom of a branched or straight-chain aliphatic hydrocarbon. | | Indications | Propylene glycol solution (40% to 60%, v/vCH2CH[OH]CH2OH, propylene glycol)
applied to the skin under plastic occlusion hydrates the skin and causes desquamation
of scales. Propylene glycol, isotonic in 2% concentration, is a widely used vehicle
in dermatologic preparations. Hydroalcoholic gels containing propylene glycol or
other substances augment the keratolytic action of salicylic acid. Keralyt gel consists
of 6% salicylic acid, 19.4% alcohol, hydroxypropylcellulose, propylene glycol, and water and is an extremely effective keratolytic agent. Overnight occlusion is used
nightly until improvement is evident, at which time the frequency of therapy can
be decreased to every third night or once weekly. This therapy is well tolerated,
is usually nonirritating, and has been most successful in patients with X-linked
ichthyosis vulgaris. Burning and stinging may occur when applied to damaged skin.
Patients with other abnormalities of keratinization with hyperkeratosis, scaling, and
dryness may also benefit. | | Production Methods | Propylene glycol generally is synthesized commercially by
starting with propylene, converting to the chlorohydrin, and
hydrolyzing to propylene oxide, which is then hydrolyzed
to propylene glycol. It can also be prepared by other
methods. | | Preparation | Manufactured by treating propylene with chlorinated water to form the chlorohydrin, which is converted to the glycol by
treatment with sodium carbonate solution. It is also prepared by heating glycerol with sodium hydroxide. | | Brand name | Sentry Propylene Glycol (Union Carbide); Sirlene (Dow Chemical). | | Aroma threshold values | Detection: 340 ppm | | General Description | Thick odorless colorless liquid. Mixes with water. | | Air & Water Reactions | Water soluble. | | Reactivity Profile | 1,2-Propanediol is hygroscopic. 1,2-Propanediol is sensitive to excessive heat (tends to oxidize at high temperatures). 1,2-Propanediol can react with oxidizing materials. 1,2-Propanediol is incompatible with acid chlorides, acid anhydrides, chloroformates, and reducing agents. 1,2-Propanediol dissolves many essential oils. A mixture of 1,2-Propanediol with hydrofluoric acid and silver nitrate was put in a glass bottle which burst 30 minutes later. | | Hazard | Toxic. | | Health Hazard | Liquid may irritate eyes. | | Fire Hazard | 1,2-Propanediol is combustible. | | Contact allergens | Propylene glycol is used as a solvent, a vehicle for
topical medicaments such as corticosteroids or aciclovir,
an emulsifier and humectant in food and cosmetics,
and as antifreeze in breweries, in the manufactures
of resins. It was present as an occupational sensitizer
in the color film developer Flexicolor?. Patch tests in
aqua are sometimes irritant. | | Safety Profile | Slightly toxic by
ingestion, skin contact, intraperitoneal,
intravenous, subcutaneous, and
intramuscular routes. Human systemic
effects by ingestion: general anesthesia,
convulsions, changes in surface EEG.
Experimental teratogenic and reproductive
effects. An eye and human skin irritant.
Mutation data reported. Combustible liquid
when exposed to heat or flame; can react
with oxidizing materials. Explosive in the
form of vapor when exposed to heat or
flame. May react with hydrofluoric acid +
nitric acid + silver nitrate to form the
explosive silver fulminate. To fight fire, use
alcohol foam. When heated to
decomposition it emits acrid smoke and
irritating fumes. | | Potential Exposure | Propylene glycol is used as a solvent;
emulsifying agent; food and feed additive; flavor, in manu-
facture of plastics; as a plasticizer, surface-active agent;
antifreeze, solvent, disinfectant, hydroscopic agent; coolant
in refrigeration systems; pharmaceutical, brake fluid; and
many others. | | Carcinogenicity | Dewhurst et al. and
Baldwin et al. in studies on the carcinogenicity of
other chemicals used propylene glycol as the solvent. As a
result they tested propylene glycol alone for carcinogenic
activity in rats and mice. Dewhurst et al. used a single
injection of 0.2 mL, whereas Baldwin et al. gave rats
and mice three to five subcutaneous injections, amount not
specified. In neither case were tumors observed during a
period of about a year or 2 years .
Wallenious and Lecholm applied propylene glycol
to the skin of rats three times a week for 14 months but found
no tumor formation. Stenback and Shubik confirmed
these findings when they applied propylene glycol at undiluted
strength and as a 50 and 10% solution in acetone to the
skin of mice during their lifetimes.
No tumors have been reported in the lifetime dietary
feeding studies . In fact, Gaunt et al.
specifically state that no tumors were found in the rats. | | Environmental Fate | Propylene glycol can be released into the environment via
industrial releases or by disposal of consumer products.
Propylene glycol is readily soluble in water and has a low
sorption partition coefficient (KOC), so has the ability to move
through soil and to leach into ground water. Because of low
vapor pressure (0.1 mmHg at 25°�C) and high water solubility,
there is minimal volatilization to the atmosphere from
surface water releases as well as substantial removal of its
vapors by wet deposition. Its low octanol/water partition
coefficient (KOW) indicates that bioconcentration and biomagnification
should not happen. Propylene glycol is readily
degraded in surface water and soil, by chemical oxidation and
microbial digestion, with a short half-life (1–5 days) in
aerobic or anaerobic conditions. It is also rapidly degraded in
the atmosphere by photochemical oxidation, with a half-life
about 1 day. Although environmental releases can and do
occur (airports must monitor storm water runoff of deicing
solutions), human health effects are likely to be minor, at
least in comparison to effects from potential exposures in
clinical scenarios. | | Purification Methods | Dry the diol with Na2SO4, decant and distil it under reduced pressure. [Beilstein 1 IV 2468.] | | Toxicity evaluation | Propylene glycol has a low degree of toxicity in animals as well
as humans, such that very high doses are needed to elicit effects
in acute toxicity studies. The toxic effects of propylene glycol
appear to be similar in animals and in humans. Central
nervous system (CNS) depression, hematologic toxicity,
hyperosmolarity, metabolic acidosis, cardiovascular effects,
and renal toxicity encompass the main acute and subacute
syndromes for propylene glycol. Most of the effects of
propylene glycol can be ascribed to high concentrations of the
parent molecule or to the accumulation of D,L-lactate in the
blood. Due to its alcohol moiety, propylene glycol at very high
concentrations is the most likely reason for the CNS depression.
Also, because high concentrations of propylene glycol will
increase the osmolarity of the blood, the hyperosmotic effects
are likely due to the parent molecule. The cardiovascular and
renal effects may be a result of the hyperosmolarity in combination
with the metabolic acidosis. The acidosis itself results
from the accumulation of lactate (both D- and L-forms), which
has been well documented in both animals and humans. | | Incompatibilities | Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explo-
sions. Keep away from alkaline materials, strong acids
(especially nitric acid), strong bases, permanganates,
dichromates; may cause a violent reaction. | | Waste Disposal | Dissolve or mix the
material with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber.All federal, state, and local environmental regulations must
be observed. |
| | Propylene glycol Preparation Products And Raw materials |
| Raw materials | Methanol-->Chlorine-->Propylene oxide-->1-Chloro-2-propanol-->Propylene carbonate | | Preparation Products | Methyl acrylate-->Pyruvic acid-->PAINT-->Phytic acid-->Adhesive-->4-Hydroxystyrene-->Unsaturated polyester resin-->1,3-Dibromopropane-->Curcumin-->2,4,5-Trichlorophenol-->1,3-Dichloropropane-->3-Chloro-1,2-propanediol-->Glycidol-->LACCAIC ACID-->Propylene carbonate-->alpha-d-Glucopyranoside, beta-d-fructofuranosyl, octadecanoate-->MONASCUS RED-->Famciclovir-->Meprobamate-->Toloxatone-->Shikonin-->Poly(propylene glycol)-->Furaneol-->PROPYLENE GLYCOL MONOSTEARATE (CONTAINS CA. 35% MONOPALMITATE)-->1,1'-Oxydi-2-propanol-->Diprophylline-->2,4-DIHYDROXY-3,3-DIMETHYLBUTANOIC ACID-->demulsifier PE series-->Maiz moraelo colour |
|