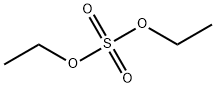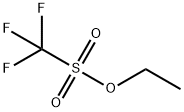
Ethyl trifluoromethanesulfonate synthesis
- Product Name:Ethyl trifluoromethanesulfonate
- CAS Number:425-75-2
- Molecular formula:C3H5F3O3S
- Molecular Weight:178.13
Yield:425-75-2 92.7%
Reaction Conditions:
at 60 - 140;Large scale;Temperature;
Steps:
3
(1) 300g of trifluoromethanesulfonic acid and 154g of diethyl sulfate were put into a 5L glass reactor, stirring was started, then the reactor was heated to 60 ° C and incubated for 2 hours. (2) The reactor was heated to 120 ~ 140 ° C, open the rectification valve, control reflux ratio of 3: 1; the same time, to the reactor by adding trifluoromethanesulfonic acid and diethyl sulfate, Sulfonic acid at a rate of 30g / min continuously added to the reactor, diethyl sulfate to 15g / min rate continuously added to the reactor, the reaction rectification, the rectification tower top temperature stable at 115 ± 3 ° C , The fractions were collected and condensed, and the purity of ethyl triflate was 99.4% in the distillate, and the product was transferred to a finished collecting tank. The trial put a total of trifluoromethanesulfonic acid 3Kg, diethyl sulfate 1. 54Kg, received the product ethyl trifluoromethanesulfonate 3. 32Kg, yield 92.7%.
References:
Zhangjiagang Guotai-Huarong New Chemical Materials Co., Ltd;Xu, Xiao Qiang;Li, Jian Zhong;Guo, Jun;Shi, suping;Qian, xiaobing;He, Yong Gang;Yu, sanbao;Yang, Sheng CN103880715, 2016, B Location in patent:Paragraph 0018; 0019
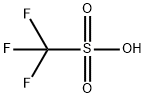
1493-13-6
597 suppliers
$12.00/5g
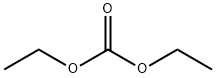
105-58-8
446 suppliers
$14.00/25g

425-75-2
221 suppliers
$20.00/1g

60-29-7
7 suppliers
$30.00/50g
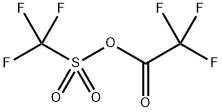
68602-57-3
66 suppliers
$16.80/100MG
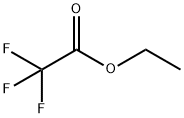
383-63-1
472 suppliers
$10.00/25 g

425-75-2
221 suppliers
$20.00/1g