Mercury-Hazard and Toxicity
Sep 9,2019
Description
Mercury is a chemical element with the symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum. A heavy, silvery d-block element, mercury is the only metallic element that is liquid at standard conditions for temperature and pressure; the only other element that is liquid under these conditions is the halogenbromine, though metals such as caesium, gallium, and rubidium melt just above room temperature. Mercury occurs in deposits throughout the world mostly as cinnabar (mercuric sulfide). The red pigment vermilion is obtained by grinding natural cinnabar or synthetic mercuric sulfide.

Mercury is used in thermometers, barometers, manometers, sphygmomanometers, float valves, mercury switches, mercury relays, fluorescent lamps and other devices, though concerns about the element's toxicity have led to mercury thermometers and sphygmomanometers being largely phased out in clinical environments in favor of alternatives such as alcohol- or galinstan-filled glass thermometers and thermistor- or infrared-based electronic instruments. Likewise, mechanical pressure gauges and electronic strain gauge sensors have replaced mercury sphygmomanometers.
Toxicity Data
LCLO inhal (rabbit) 29 mg/m3 (30 h)
PEL (OSHA) 0.1 mg/m3 (ceiling)
TLV-TWA (ACGIH) 0.025 mg/m3—skin
Major Hazards
Repeated or prolonged exposure to mercury vapor is highly toxic to the central nervous system. Mercury is a non-specific toxin, attacking many of the body s systems. At low levels of exposure, symptoms are mainly related to nerve and brain function and include memory loss, mood instability, tremor, and other stress-like symptoms: poor coordination, headache, and visual and hearing problems. Recently, reproductive health has been shown to be affected, with abnormalities in menstrual cycle, poor outcome of pregnancy, and subfertility in both men and women. The immune system is also damaged by mercury exposure.
Toxicity
The acute toxicity of mercury varies significantly with the route of exposure. Ingestion is largely without effects. Inhalation of high concentrations of mercury causes severe respiratory irritation, digestive disturbances, and marked kidney damage. There are no warning properties for exposure to mercury vapor, which is colorless, odorless, and tasteless.
Toxicity caused by repeated or prolonged exposure to mercury vapor or liquid is characterized by emotional disturbances, inflammation of the mouth and gums, general fatigue, memory loss, headaches, tremors, anorexia, and weight loss. Skin absorption of mercury and mercury vapor adds to the toxic effects of vapor inhalation. At low levels the onset of symptoms is insidious; fine tremors of the hand, eyelids, lips, and tongue are often the presenting complaints. Mercury has been reported to be capable of causing sensitization dermatitis. Mercury has not been shown to be a human carcinogen or reproductive toxin.
Reactivity and Incompatibility
Mercury is a fairly unreactive metal that is highly resistant to corrosion. It can dissolve a number of metals, such as silver, gold, and tin, forming amalgams. Mercury can react violently with acetylene and ammonia.
Storage and Handling
In particular, precautions should be taken to prevent spills of mercury because drops of the liquid metal can easily become lodged in floor cracks, behind cabinets, and equipment, etc., with the result that the mercury vapor concentration in the laboratory may then exceed the safe and allowable limits.
Containers of mercury should be kept tightly sealed and stored in secondary containers (such as a plastic pan or tray) in a well-ventilated area. When breakage of instruments or apparatus containing significant quantities of Hg is possible, the equipment should be placed in a plastic tray or pan that is large enough to contain the mercury in the event of an accident. Transfers of mercury between containers should be carried out in a fume hood over a tray or pan to confine any spills.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If mercury is ingested, obtain medical attention immediately. If large amounts of this substance are inhaled, move the person to fresh air and seek medical attention at once. Respiratory protection will be necessary in the event of a large spill, release in a confined area, or spill under conditions of higher than normal temperatures.
Disposal
Excess mercury should be collected for recycling, and waste material containing mercury should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
- Related articles
- Related Qustion
- Mercury metal: Sources and Applications May 11, 2024
Mercury is a naturally occurring metallic chemical element.
- Mercury: Applications, metabolism and toxicity Jun 26, 2023
Mercury is a chemical element with the symbol Hg and atomic number 80. It is commonly known as quicksilver and was formerly named hydrargyrum.
- Mercury - History and Drug-Application Oct 19, 2020
Since around 500 BC, the mines at Sisapo in Almade′n (Arabic for the mine) in Southern Spain have been the principal source of Iberian cinnabar, red mercuric sulfide.
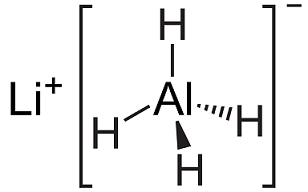
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula LiAlH4. It was discovered by Finholt, Bond and Schlesinger in 1947. This compound is used as a reducing agent in organic synthesis, e....
Sep 9,2019Inorganic chemistryMethyl alcohol, also known as methanol or wood alcohol, is a clear, colorless, flammable liquid that is the simplest alcohol. World production of methanol is approximately 8.5 billion gallons annually. Methanol is produced industrially, sta....
Sep 9,2019Organic reagentsMercury
7439-97-6You may like
- MercuryMercury
-

- $6.00 / 1KG
- 2025-03-14
- CAS:07439-97-6
- Min. Order: 1KG
- Purity: More than 99%
- Supply Ability: 500KG/Month
- Sliver Mercury
-

- $190.00/ kg
- 2024-12-02
- CAS:7439-97-6
- Min. Order: 345kg
- Purity: 99.9%
- Supply Ability: 5 Ton
- Liquid Mercury
-

- $190.00/ kg
- 2024-12-02
- CAS:7439-97-6
- Min. Order: 100kg
- Purity: 99.9%
- Supply Ability: 5 Ton