Titanium Tetrachloride: Stepping Stone to Amazing Technology
Nov 15,2019
What do spacecraft, bicycles and artificial knee replacements have in common? All are made better with titanium metal. The chemical element titanium, Ti, number 22 on the Periodic Table of the Elements, is the ninth most abundant element in the Earth's crust. It is found in meteorites, moon rocks and the sun. Titanium is as strong as steel, but 45 percent lighter. As a high-strength, heat-resistant, low-density metal that resists corrosion, titanium does the job when lightweight durability is required.
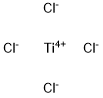
On Earth, titanium occurs naturally, but only combined chemically with other elements in compounds. It appears, for example, in the mineral rutile, titanium dioxide (TiO2). In 1946, a scientist named William Kroll invented a process to remove, or extract, titanium from titanium dioxide. Kroll used chlorine in an intermediate step that temporarily created titanium tetrachloride, TiCl4, sometimes humorously called "tickle." The Kroll Process is still in use today.

From Sands to Spacecraft
Titanium-containing minerals, such as rutile, are mined from mineral sand deposits. If you have ever looked at beach sands through a magnifying glass, you might have noticed that they are made of very tiny pieces of minerals, such as quartz. The mineral pieces are very small because they have been ground down over millions of years by wind and water.
Titanium-rich sands are economically valuable as they contain a small percentage of titanium minerals, such as rutile. In the U.S., titanium-rich sands are mined in Florida and Virginia. Globally, the U.S., India, Australia and South Africa are some of the major producers of titanium sands.
The process of extracting titanium-rich sand from mineral sand deposits is not destructive to the landscape. After titanium-rich grains are separated from the other grains, the remaining sand is replaced and re-shaped into a natural form.
The Kroll Process for Extracting Titanium Metal
Chemical manufacturing processes are carried out in steps, just as bakers follow recipes for cookies and cakes in steps (for best results, that is!). These are the steps that Kroll devised for extracting titanium from titanium dioxide:
(1) Combine titanium dioxide (titanium ore, the mineral sand deposit described above) with chlorine gas and carbon (called "coke") at 1,000 degrees C to produce titanium tetrachloride, a yellow liquid, and carbon dioxide gas:
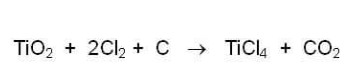
(2) Combine titanium tetrachloride liquid with magnesium metal at 800-900 degrees C to replace titanium in the chloride compound, and release titanium metal:
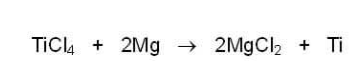
Step (2), which replaces titanium with magnesium, is known as a metal displacement reaction. It is carried out in an argon (Ar) atmosphere, meaning that the only gas in the reaction chamber is argon, which will not interfere with the reaction. (Oxygen, on the other hand, would react with titanium to make titanium dioxide, so it is pumped out as argon is pumped in.) Notice that titanium tetrachloride is not a product of the Kroll Process, but an important stepping-stone, or intermediate compound.
Speaking of sandy beaches, perhaps you've been at the beach when a message, possibly an ad for a sunscreen product, has been delivered overhead by a sky-writing airplane. "Tickle," TiCl4 is sprayed into the air to create this message. Here's the chemical reaction:
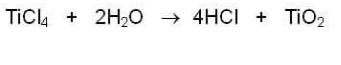
You can probably guess where the H2O comes from-yes, the moisture in the seaside air. The titanium dioxide forms tiny white crystals that you see as the sky-writing "ink."
Versatile TiO2
As important a metal as titanium is, the major use of titanium in the world is as titanium dioxide. TiO2 is a pigment, commonly added to house paints and artists' paints to impart a brilliant, highly reflective, permanent white color. Non-toxic, titanium dioxide replaced lead, a toxic element, as the traditional white pigment used in paints. TiO2 also provides UV protection, so it's found in sunscreens (you can take it to the beach where you can lie on a Ti-rich sand dune and gaze at TiCl4 sky-writing), toothpaste and cosmetics, and many other products we use every day.

Like titanium metal, very pure titanium dioxide can also be produced using chlorine to form titanium tetrachloride from impure titanium dioxide ore. It is then combined with oxygen, which releases chlorine. The chlorine is "captured" and recycled as it is used to form more TiCl4 from ore.
- Related articles
- Related Qustion
Iodine monochloride (ICl) was discovered to be a highly efficient, green oxidant, which can oxidize aldose hemiacetals, diarylmethanols, arylalkylmethanols, anddialkylmethanols to the corresponding aldose lactones, diarylmethanones, arylalk....
Nov 15,2019Inorganic chemistryZinc chloride is the name of chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water.....
Nov 15,2019APITitanium tetrachloride
7550-45-0You may like
Titanium tetrachloride manufacturers
- Titanium tetrachloride
-

- $10.00 / 1kg
- 2025-12-11
- CAS:7550-45-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100 mt
- Titanium(IV) chloride
-

- $6000.00 / 1T
- 2025-06-20
- CAS:7550-45-0
- Min. Order: 1T
- Purity: 99%
- Supply Ability: 20tons
- Titanium tetrachloride
-

- $0.00 / 1kg
- 2025-06-20
- CAS:7550-45-0
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 100tons