| Identification | More | [Name]
Phenol | [CAS]
108-95-2 | [Synonyms]
AKOS BBS-00004229
BENZENOL
BIOPHENOL
CARBOLIC ACID
CARBOLIC ACID SOLID
FEMA 3223
HYDROXYBENZENE
HYDROXY BENZENE SOLID
KJELDAHL PHENOL REAGENT
LIQUIFIED PHENOL
OXYBENZENE
PHENIC ACID
PHENOL
PHENOL, LIQUEFIED
PHENOL LIQUIFIED
PHENOL REAGENT
PHENOL SATURATED, PH 4.5
PHENOL SINGLE PHASE BUFFER, SATURATED
PHENOL SOLUTION
PHENOL STANDARD | [EINECS(EC#)]
203-632-7 | [Molecular Formula]
C6H6O | [MDL Number]
MFCD00002143 | [Molecular Weight]
94.11 | [MOL File]
108-95-2.mol |
| Chemical Properties | Back Directory | [Description]
Phenol is a stable chemical substance and appear as colourless/white crystals with a
characteristic, distinct aromatic/acrid odour. It is reactive and incompatible with strong
oxidising agents, strong bases, strong acids, alkalis, and calcium hypochlorite. Phenol is
flammable and may discolour in light.
Phenol is used in the manufacture or production of explosives, fertiliser, coke, illuminating
gas, lampblack, paints, paint removers, rubber, perfumes, asbestos goods, wood
preservatives, synthetic resins, textiles, drugs, and pharmaceutical preparations. It is also
extensively used as a disinfectant in the petroleum, leather, paper, soap, toy, tanning, dye,
and agricultural industries. | [Appearance]
Phenol is a colorless to light-pink, crystalline solid. Sweet, acrid odor. Phenol liquefies by mixing with about 8% water. The Odor Threshold in air is 0.04 ppm and in water is 7.9 ppm. | [Melting point ]
40-42 °C(lit.)
| [Boiling point ]
182 °C(lit.)
| [bulk density]
620kg/m3 | [density ]
1.071 g/mL at 25 °C(lit.)
| [vapor density ]
3.24 (vs air)
| [vapor pressure ]
0.09 psi ( 55 °C)
| [FEMA ]
3223 | [refractive index ]
n20/D 1.53
| [Fp ]
175 °F
| [storage temp. ]
2-8°C
| [solubility ]
H2O: 50 mg/mL at 20 °C, clear, colorless
| [form ]
liquid
| [pka]
9.89(at 20℃) | [color ]
faintly yellow
| [Specific Gravity]
1.071 | [Odor]
Sweet, medicinal odor detectable at 0.06 ppm | [PH]
3.0-6.0 (25℃, 0.5M in H2O) | [biological source]
synthetic | [explosive limit]
1.3-9.5%(V) | [Odor Threshold]
0.0056ppm | [Odor Type]
phenolic | [Water Solubility ]
8 g/100 mL | [FreezingPoint ]
41℃ | [Sensitive ]
Air & Light Sensitive | [Usage]
Purified for molecular genetics applications | [JECFA Number]
690 | [Merck ]
14,7241 | [BRN ]
969616 | [Henry's Law Constant]
1.09 at 5 °C (average derived from six field experiments, Lüttke and Levsen, 1997) | [Dielectric constant]
4.3(10℃) | [Exposure limits]
TLV-TWA skin 5 ppm (~19 mg/m3 )
(ACGIH, MSHA, and OSHA); 10-hour TWA 5.2 ppm (~20 mg/m3 ) (NIOSH); ceiling
60 mg (15 minutes) (NIOSH); IDLH 250
ppm (NIOSH). | [Stability:]
Hygroscopic | [Major Application]
agriculture
cleaning products
cosmetics
environmental
food and beverages
personal care | [Cosmetics Ingredients Functions]
PRESERVATIVE
ORAL CARE
FRAGRANCE
DENATURANT
ANTIMICROBIAL
DEODORANT | [InChI]
1S/C6H6O/c7-6-4-2-1-3-5-6/h1-5,7H | [InChIKey]
ISWSIDIOOBJBQZ-UHFFFAOYSA-N | [SMILES]
Oc1ccccc1 | [LogP]
1.47 at 30℃ | [Uses]
phenol is frequently used for medical chemical face peels. It may trap free radicals and can act as a preservative. Phenol, however, is an extremely caustic chemical with a toxicity potential. It is considered undesirable for use in cosmetics. even at low concentrations, it frequently causes skin irritation, swelling, and rashes. | [CAS DataBase Reference]
108-95-2(CAS DataBase Reference) | [IARC]
3 (Vol. 47, 71) 1999 | [NIST Chemistry Reference]
Phenol(108-95-2) | [EPA Substance Registry System]
108-95-2(EPA Substance) | [Absorption]
cut-off at 294nm in H2O at 0.5M |
| Hazard Information | Back Directory | [Chemical Properties]
Phenol is a stable chemical substance of colorless/white crystals with a characteristically
distinct aromatic/acrid odor. It is reactive and incompatible with strong oxidizing agents,
strong bases, strong acids, alkalis, and calcium hypochlorite. It is flammable and discolors
in light. Phenol is used in the manufacture or production of explosives, fertilizer, coke, illuminating
gas, lampblack, paints, paint removers, rubber, perfumes, asbestos goods, wood
preservatives, synthetic resins, textiles, drugs, and pharmaceutical preparations. It is also
used extensively as a disinfectant in the petroleum, leather, paper, soap, toy, tanning, dye,
and agricultural industries. Phenol is a systemic poison and constitutes a serious health
hazard. The risks of using it in the laboratory must be fully assessed before work begins.
Typical MEL 2 ppm; typical OEL 1 ppm. | [General Description]
A solid melting at 110°F. Colorless if pure, otherwise pink or red. Flash point 175°F. Density 9.9 lb/gal. Vapors are heavier than air Corrosive to the skin (turning skin white) but because of its anesthetic quality numbs rather than burn. Lethal amounts can be absorbed through the skin. Used to make plastics and adhesives. | [Reactivity Profile]
PHENOL is a weak acid. Reacts exothermically with bases. Reacts with strong oxidizing agents. Emits acrid smoke and irritating fumes when heated to decomposition. Undergoes, in the presence of aluminum chloride, potentially explosive reactions with nitromethane, butadiene, formaldehyde, peroxodisulfuric acid, peroxosulfuric acid, and sodium nitrite . Reacts violently with sodium nitrate in the presence of trifluoroacetic acid [Bretherick, 5th ed., 1995, p. 770]. May corrode lead, aluminum and its alloys, certain plastics, and rubber. Phenol may explode in contact with peroxodisulfuric acid (Dns, J. Ber., 1910, 43, 1880; Z. Anorg. Chem., 1911, 73, 1911.) or peroxomonosulfuric acid. (Sidgwick, 1950, 939) | [Air & Water Reactions]
Decomposes slowly in air. Mixtures of 9-10% phenol in air are explosive. Soluble in water | [Health Hazard]
Exposures to phenol cause adverse health effects and poisoning. Phenol is absorbed very rapidly
through surfaces of the skin, lungs, and stomach. The symptoms of prolonged exposures
and poisoning include, but are not limited to, vomiting, diffi culty in swallowing, diarrhea,
lack of appetite, headache, fainting, dizziness, mental disturbances, and skin rash. Direct contact
with phenol causes burning of the mouth, irritation to the eyes, nose, and dermatitis,
discoloration of the skin, and damage to the liver and kidneys. Exposure to phenol in different
concentrations is known to cause mental disturbances, depression of the CNS, and coma. | [Health Hazard]
Toxic hazard rating is very toxic: probable oral lethal dose (human) is 50-500 mg/kg. Ingestion of 1 gram has been lethal to humans. Lethal amounts may be absorbed through skin or inhaled. Industrial contact can cause chronic poisoning with kidney and liver damage. Persons affected with hepatic or kidney diseases are at a greater risk. | [Potential Exposure]
Phenol is used as a pharmaceutical, in the production of fertilizer; coke, illuminating gas; lampblack, paints, paint removers; rubber, asbestos goods; wood preservatives; synthetic resins; textiles, drugs, pharmaceutical preparations; perfumes, bakelite, and other plastics (phenolformaldehyde resins); polymer intermediates (caprolactam, bisphenol-A and adipic acid). Phenol also finds wide use as a disinfectant and veterinary drug. | [Fire Hazard]
Flammable vapors when heated. Runoff from fire control water may give off poisonous gases and cause pollution. Mixtures of 9-10% phenol in air are explosive. Avoid aluminum chloride/nitrobenzene mixture, peroxodisulfuric acid, peroxomonosulfuric acid and strong oxidizing agents. Decomposes slowly on air contact. Avoid contact with strong oxidizing agents. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. If concentrated phenol gets on a large area of the skin, immediately rush victim to shower and use at full blast; remove all contaminated clothing; scrub the contaminated area with soap for at least 10 minutes—water alone may be harmful. If polyethyleneglycol-300 is available, swab exposed area with cotton soaked in it. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Rinse mouth. Give plenty of water and/or vegetable oil to drink. Do not allow the consumption of alcohol. Induce vomiting. Do not make an unconscious person vomit. Medical observation is recommended for 24�48 hours after breathing overexposure, as pulmonary edema may be delayed. As first aid for pulmonary edema, a doctor or authorized paramedic may consider administering a drug or other inhalation therapy. | [Shipping]
UN1671 Phenol, solid, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2312 Molten phenol, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. UN2821 Phenol solutions, Hazard Class: 6.1; Labels: 6.1-Poisonous materials. | [Incompatibilities]
Vapors may form explosive mixture with air. The aqueous solution is a weak acid. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, calcium hypochlorite; aluminum chloride. acids. Reacts with metals. | [Waste Disposal]
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration. | [Physical properties]
Phenol is a colorless or white crystalline solid that is slightly soluble in water. Phenol is the
simplest of the large group of organic chemicals known as phenols, which consist of compounds
where a carbon in the phenyl aromatic group (C6H5) is directly bonded to hydroxyl,
OH. | [Occurrence]
It is reported found in over 150 natural products including apricot, sour cherry, black currant, bilberry,
cranberry, other berries, grapes, guava fruit, peach, pineapple, asparagus, onion, cooked potato, tomato, cinnamon bark, cassia
leaf, ginger, pennyroyal oil, many cheeses, butter, milk, milk powder, boiled egg, fish and fish oil, cooked and cured meats, beer,
wheaten bread, crisp bread, cognac, rose wine, cocoa, coffee, tea, whiskies, roasted filbert, roasted peanut, soybean, pecans,
honey, avocado, Arctic bramble, passion fruit, beans, mushrooms, burley tobacco, cooked beef and chicken, fermented soy sauce,
trassi, roasted almonds, sesame seed, fenugreek, mango, tamarind, Brazil nut, rice, rhubarb, licorice, buckwheat, watercress, malt,
wort, dried bonito, loquat, myrtle berry, rosemary, Tahiti and Bourbon vanilla, endive, shrimp, crab, crayfish, clam, squid, truffle
and Chinese quince. | [History]
Phenol’s first prominent use was by Joseph Lister (1827–1912) as an antiseptic.
Throughout human history,infection often resulted in death,even when the wound could
be surgically treated.A broken bone piercing the skin, which today is a painful but not
life-threatening injury,historically resulted in infection and possible amputation or death.
Lister was inspired by Louis Pasteur’s (1822–1895) germ theory of disease,and he began
to use antiseptic methods during routine surgery during the 1860s. | [Definition]
1. (carbolic acid,
hydroxybenzene, C6H5OH) A white crystalline
solid used to make a variety of other
organic compounds.
2. A type of organic compound in which at
least one hydroxyl group is bound directly
to one of the carbon atoms of an aromatic
ring. Phenols do not show the behavior
typical of alcohols. In particular they are
more acidic because of the electron-withdrawing
effect of the aromatic ring. The
preparation of phenol itself is by fusing the
sodium salt of the sulfonic acid with
sodium hydroxide:
C6H5SO2.ONa + 2NaOH → C6H5ONa
+ Na2SO3 + H2O
The phenol is then liberated by sulfuric
acid:
2C6H5ONa + H2SO4 → 2C6H5OH +
Na2SO4
Reactions of phenol include:
1. Replacement of the hydroxyl group with
a chlorine atom using phosphorus(V)
chloride.
2. Reaction with acyl halides to form esters
of carboxylic acids.
3. Reaction with haloalkanes under alkaline
conditions to give mixed alkyl–aryl
ethers.
In addition phenol can undergo further
substitution on the benzene ring. The hydroxyl
group directs other substituents
into the 2- and 4-positions. | [Definition]
ChEBI: An organic hydroxy compound that consists of benzene bearing a single hydroxy substituent. The parent of the class of phenols. | [Indications]
Phenol in dilute solution (0.5% to 2%) decreases itch by anesthetizing the cutaneous
nerve endings. Phenol should never be used on pregnant women or infants younger
than 6 months of age. | [Preparation]
Phenol is formed in dry distillation of wood, peat and coal; coal tar is one of the commercial sources of phenol and its
homologues. | [Production Methods]
Historically, phenol was produced by the distillation of coal tar.
Today, phenol is prepared by one of several synthetic methods, such
as the fusion of sodium benzenesulfonate with sodium hydroxide
followed by acidification; the hydrolysis of chlorobenzene by dilute
sodium hydroxide at high temperature and pressure to give sodium
phenate, which on acidification liberates phenol (Dow process); or
the catalytic vapor-phase reaction of steam and chlorobenzene at
500°C (Raschig process). | [Production Methods]
Phenol was prepared before World War I through the distillation of coal tar. The firstsynthetic process involved the sulfonation of benzene followed by desulfonation with abase.
The most common current method of phenol production is from the cumene hydroperoxiderearrangement process.In this process,benzene reacts with propylene to produce cumene.Cumene is oxidized to cumene hydroperoxide.When cumene hydroperoxide is treated withdilute sulfuric acid,it rearranges and splits into phenol and acetone. Because the reactants areinexpensive and the process is simple,the acidic oxidation of cumene is used to produce morethan 95% of the world’s supply of phenol. | [World Health Organization (WHO)]
Phenol became widely used as an antiseptic following
demonstration of its germicidal activity in 1867. It is an intensely corrosive
substance and percutaneous absorption can produce serious systemic toxicity. It
has been withdrawn from pharmaceutical preparations by at least one national
regulatory authority. However, it is still used widely in concentrations of the order
of 1.4% in proprietary preparations for the relief of soreness of the mouth and
throat. | [Aroma threshold values]
Detection: 5.5 ppm. Aroma characteristics at 1.0%: medicinal, creosote, smoky, spicy, phenolic, leatherlike
with notes of fried meat and coffee. | [Taste threshold values]
Taste characteristics at 3 ppm: spicy, phenolic, tobacco, musty, woody, medicinal, smoky, tarlike and
slightly spicy clovelike. | [Synthesis Reference(s)]
Journal of the American Chemical Society, 107, p. 2153, 1985 DOI: 10.1021/ja00293a054
Synthetic Communications, 19, p. 453, 1989 DOI: 10.1080/00397918908050686 | [Flammability and Explosibility]
Phenol is a combustible solid (NFPA rating = 2). When heated, phenol produces
flammable vapors that are explosive at concentrations of 3 to 10% in air. Carbon
dioxide or dry chemical extinguishers should be used to fight phenol fires. | [Pharmaceutical Applications]
Phenol is used mainly as an antimicrobial preservative in parenteral
pharmaceutical products. It has also been used in topical
pharmaceutical formulations and cosmetics;
Phenol is widely used as an antiseptic, disinfectant, and
therapeutic agent, although it should not be used to preserve
preparations that are to be freeze-dried. | [Industrial uses]
Phenol is the simplest member of a class oforganic compounds possessing a hydroxylgroup attached to a benzene ring or to a morecomplex aromatic ring system.
Also known as carbolic acid or monohydroxybenzene,phenol is a colorless to whitecrystalline material of sweet odor, having thecomposition C6H5OH, obtained from the distillationof coal tar and as a by-product ofcoke ovens.
Phenol has broad biocidal properties, anddilute aqueous solutions have long been usedas an antiseptic. At higher concentrations itcauses severe skin burns; it is a violent systemicpoison. It is a valuable chemical raw materialfor the production of plastics, dyes, pharmaceuticals,syntans, and other products.
Phenol is one of the most versatile industrialorganic chemicals. It is the starting point formany diverse products used in the home andindustry. A partial list includes nylon, epoxyresins, surface active agents, synthetic detergents,plasticizers, antioxidants, lube oil additives,phenolic resins (with formaldehyde, furfural,and so on), cyclohexanol, adipic acid,polyurethanes, aspirin, dyes, wood preservatives,herbicides, drugs, fungicides, gasolineadditives, inhibitors, explosives, and pesticides. | [Biochem/physiol Actions]
Phenol?has the ability to denature protein, hence can lead to denervation. At lower concentration, it can serve as a local anaesthetic and can also act as a neurolytic agent in higher concentration. It is also linked with tissue damage at higher concentrations. | [Safety]
Phenol is highly corrosive and toxic, the main effects being on the
central nervous system. The lethal human oral dose is estimated to
be 1 g for an adult.
Phenol is absorbed from the gastrointestinal tract, skin, and
mucous membranes, and is metabolized to phenylglucuronide and
phenyl sulfate, which are excreted in the urine.
Although there are a number of reports describing the toxic
effects of phenol, these largely concern instances of accidental
poisoning or adverse reactions during its use as a therapeutic
agent.Adverse reactions associated with phenol used as a
preservative are less likely owing to the smaller quantities that are
used; however, it has been suggested that the body burden of phenol
should not exceed 50 mg in a 10-hour period.This amount could
be exceeded following administration of large volumes of phenolpreserved
medicines.
LD50 (mouse, IV): 0.11 g/kg
LD50 (mouse, oral): 0.3 g/kg
LD50 (rabbit, skin): 0.85 g/kg
LD50 (rat, skin): 0.67 g/kg
LD50 (rat, oral): 0.32 g/kg
LD50 (rat, SC): 0.46 g/kg | [Carcinogenicity]
Phenol had been investigated for carcinogenicity in animals by the oral and dermal routes. IARC and IRIS determined that animal human evidence for carcinogenicity was inadequate. | [Source]
Detected in distilled water-soluble fractions of 87 octane unleaded gasoline (1.53 mg/L),
94 octane unleaded gasoline (0.19 mg/L), Gasohol (0.33 mg/L), No. 2 fuel oil (0.09 mg/L), jet fuel
A (0.09 mg/L), diesel fuel (0.07 mg/L), and military jet fuel JP-4 (0.22 mg/L) (Potter, 1996).
Phenol was also detected in 80% of 65 gasoline (unleaded regular and premium) samples (62 from
Switzerland, 3 from Boston, MA). At 25 °C, phenol concentrations ranged from 63 to 130,000
μg/L in gasoline and from 150 to 1,500 μg/L in water-soluble fractions. Average concentrations
were 26 mg/L in gasoline and 6.1 mg/L in water-soluble fractions (Schmidt et al., 2002).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
625. Average phenol concentrations reported in water-soluble fractions of unleaded gasoline,
kerosene, and diesel fuel were 20, 8, and 19 μg/L, respectively.
A high-temperature coal tar contained phenol at an average concentration of 0.61 wt %
(McNeil, 1983).
Phenol occurs naturally in many plants including blueberries (10 to 60 ppb), marjoram (1,431–
8,204 ppm), sweetflag, safflower buds (40 ppb), mud plantain, capillary wormwood, asparagus shoots, tea leaves, petitgrain, cinnamon, cassia, licorice, witch hazel, Japanese privet, St. John’s
wort, European pennyroyal, tomatoes, white mulberries, tobacco leaves, benneseed, sesame seeds,
tamarind, white sandlewood, patchouli leaves, rue, slash pine, bayberries, Scotch pine, and
tarragon (Duke, 1992).
A liquid swine manure sample collected from a waste storage basin contained phenol at a
concentration of 22.0 mg/L (Zahn et al., 1997).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rates of phenol were 525 mg/kg of pine burned, 300 mg/kg of oak burned, and 434 mg/kg of
eucalyptus burned.
Releases toxic and noxious fumes when heated at temperatures greater than its boiling point.
Drinking water standard: No MCLGs or MCLs have been proposed, however, a DWEL of 20
mg/L was recommended (U.S. EPA, 2000). | [Environmental Fate]
Biological. Under methanogenic conditions, inocula from a municipal sewage treatment plant
digester degraded phenol to carbon dioxide and methane (Young and Rivera, 1985).
Chloroperoxidase, a fungal enzyme isolated from Caldariomyces fumago, reacted with phenol
forming 2- and 4-chlorophenol, the latter in a 25% yield (Wannstedt et al., 1990). In activated
sludge, 41.4% mineralized to carbon dioxide after 5 d (Freitag et al., 1985). When phenol was
statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater
inoculum, significant biodegradation with rapid adaptation was observed. At concentrations of 5
and 10 mg/L, 96 and 97% biodegradation, respectively, were observed after 7 d (Tabak et al.,
1981). Phenol is rapidly degraded in aerobically incubated soil but is much slower under anaerobic
conditions (Baker and Mayfield, 1980).
Soil. Loehr and Matthews (1992) studied the degradation of phenol in different soils under
aerobic conditions. In a slightly basic sandy loam (3.25% organic matter) and in acidic clay soil
(<1.0% organic matter), the resultant degradation half-lives were 4.1 and 23 d, respectively.
Soil sorption distribution coefficients (Kd) were determined from centrifuge column tests using
kaolinite as the absorbent (Celorie et al., 1989). Values for Kd ranged from 0.010 to 0.054 L/g.
Surface Water. Vaishnav and Babeu (1987) reported a half-life of 11 d in river waters and 3 d in
harbor waters.
Groundwater. Nielsen et al. (1996) studied the degradation of phenol in a shallow, glaciofluvial,
unconfined sandy aquifer in Jutland, Denmark. As part of the in situ microcosm study, a cylinder
that was open at the bottom and screened at the top was installed through a cased borehole
approximately 5 m below grade. Five liters of water was aerated with atmospheric air to ensure
aerobic conditions were maintained. Groundwater was analyzed weekly for approximately 3
months to determine phenol concentrations with time. The experimentally determined first-order
biodegradation rate constant and corresponding half-life were 0.5/d and 33.4 h, respectively.
Vaishnav and Babeu (1987) reported a biodegradation rate constant of 0.035/d and a half-life of 20
d in groundwater.
Photolytic. Absorbs UV light at a maximum wavelength of 269 nm (Dohnal and Fenclová,
1995). In an aqueous, oxygenated solution exposed to artificial light (λ = 234 nm), phenol was
photolyzed to hydroquinone, catechol, 2,2 -, 2,4 - and 4,4 -dihydroxybiphenyl (Callahan et al.,
1979). When an aqueous solution containing potassium nitrate (10 mM) and phenol (1 mM) was
irradiated with UV light (λ = 290–350 nm) up to a conversion of 10%, the following products
formed: hydroxyhydroquinone, hydroquinone, resorcinol, hydroxybenzoquinone, benzoquinone,
catechol, nitrosophenol, 4-nitrocatechol, nitrohydroquinone, 2- and 4-nitrophenol. Catechnol and
hydroquinone were the major and minor products, respectively (Niessen et al., 1988). Titanium
dioxide suspended in an aqueous solution and irradiated with UV light (λ = 365 nm) converted
phenol to carbon dioxide at a significant rate (Matthews, 1986).
Chemical/Physical. In an environmental chamber, nitrogen trioxide (10,000 ppb) reacted
quickly with phenol (concentration 200 ppb to 1.4 ppm) to form phenoxy radicals and nitric acid
(Carter et al., 1981). The phenoxy radicals may react with oxygen and nitrogen dioxide to form
quinones and nitrohydroxy derivatives, respectively (Nielsen et al., 1983). | [storage]
When exposed to air and light, phenol turns a red or brown color,
the color being influenced by the presence of metallic impurities.
Oxidizing agents also hasten the color change. Aqueous solutions of
phenol are stable. Oily solutions for injection may be sterilized in
hermetically sealed containers by dry heat. The bulk material
should be stored in a well-closed, light-resistant container at a
temperature not exceeding 15°C. | [Purification Methods]
Steam is passed through a boiling solution containing 1mole of phenol and 1.5-2.0moles of NaOH in 5L of H2O until all non-acidic material has distilled. The residue is cooled, acidified with 20% (v/v) H2SO4, and the phenol is separated, dried with CaSO4 and fractionally distilled under reduced pressure. It is then fractionally crystallised several times from its melt [Andon et al. J Chem Soc 5246 1960]. Purification via the benzoate has been used by Berliner, Berliner and Nelidow [J Am Chem Soc 76 507 1954]. The benzoate,(m 70o, b 314o/760mm), is crystallised from 95% EtOH, then hydrolysed to the free phenol by refluxing with two equivalents of KOH in aqueous EtOH until the solution becomes homogeneous. It is acidified with HCl and extracted with diethyl ether. The ether layer is freed from benzoic acid by thorough extraction with aqueous NaHCO3, and, after drying and removing the ether, the phenol is distilled. Phenol has also been crystallised from a 75% w/w solution in water by cooling to 11o and seeding with a crystal of the hydrate. The crystals are centrifuged off, rinsed with cold water (0-2o), saturated with phenol, and dried. It can be crystallised from pet ether [Berasconi & Paschalis J Am Chem Soc 108 2969 1986]. Draper and Pollard [Science 109 448 1949] added 12% water, 0.1% aluminium (can also use zinc) and 0.05% NaHCO3 to phenol, and distilled it at atmospheric pressure until the azeotrope was removed, The phenol was then distilled at 25mm. Phenol has also been dried by distillation from the *benzene solution to remove the water/*benzene azeotrope and the excess *benzene, followed by distillation of the phenol at reduced pressure under nitrogen. Processes such as this are probably adequate for analytical grade phenol which has as its main impurity water. Phenol has also been crystallised from pet ether/*benzene or pet ether (b 40-60o). The purified material is stored in a vacuum desiccator over P2O5 or CaSO4. [Beilstein 6 IV 531.] | [Toxics Screening Level]
The initial threshold screening level (ITSL) for phenol is 190 μg/m3 based on an 8-hour averaging time. | [Regulatory Status]
Included in the FDA Inactive Ingredients Database (injections).
Included in medicines licensed in the UK. Included in the Canadian
List of Acceptable Non-medicinal Ingredients. |
| Safety Data | Back Directory | [Hazard Codes ]
T,C,F,Xn | [Risk Statements ]
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed .
R34:Causes burns.
R48/20/21/22:Harmful: danger of serious damage to health by prolonged exposure through inhalation, and in contact with skin and if swallowed .
R68:Possible risk of irreversible effects.
R40:Limited evidence of a carcinogenic effect.
R39/23/24/25:Toxic: danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed .
R11:Highly Flammable.
R36:Irritating to the eyes.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R24/25:Toxic in contact with skin and if swallowed . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S36/37:Wear suitable protective clothing and gloves .
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes .
S1/2:Keep locked up and out of the reach of children .
S36:Wear suitable protective clothing .
S16:Keep away from sources of ignition-No smoking . | [OEB]
A | [OEL]
TWA: 5 ppm (19 mg/m3), Ceiling: 15.6 ppm (60 mg/m3) [15-minute] [skin] | [RIDADR ]
UN 2821 6.1/PG 2
| [WGK Germany ]
2
| [RTECS ]
SJ3325000
| [F ]
8-23 | [Autoignition Temperature]
715 °C | [TSCA ]
Yes | [HazardClass ]
6.1 | [PackingGroup ]
II | [HS Code ]
29071100 | [Storage Class]
3 - Flammable liquids | [Hazard Classifications]
Acute Tox. 4 Dermal
Acute Tox. 4 Inhalation
Acute Tox. 4 Oral
Eye Irrit. 2
Flam. Liq. 2 | [Precautions]
Acute poisoning of phenol by ingestion, inhalation or skin contact may lead to death.
Phenol is readily absorbed through the skin. It is highly toxic by inhalation. It is corrosive
and causes burns and severe irritation effects. During use and handling of phenol, occupational
workers should be very careful. Workers should use protective clothing, rubber
boots, and goggles to protect the eyes from vapors and spillage. | [Safety Profile]
Human poison by ingestion. An experimental poison by ingestion, subcutaneous, intravenous, parenteral, and intraperitoneal routes. Moderately toxic by skin contact. A severe eye and skin irritant. Questionable carcinogen with experimental carcinogenic and neoplastigenic data. Mutation data reported. An experimental teratogen. Absorption of phenolic solutions through the skin may be very rapid, and can cause death within 30 minutes to several hours by exposure of as little as 64 square inches of skin. Lesser exposures can cause damage to the ladneys, liver, pancreas, and spleen, and edema of the lungs. Ingestion can cause corrosion of the lips, mouth, throat, esophagus, and stomach, and gangrene. Ingestion of 1.5 g has lulled. Chronic exposures can cause death from liver and kidney damage. Dermatitis resulting from contact with phenol or phenol-containing products is fairly common in industry. A common air contaminant.Combustible when exposed to heat, flame or oxidizers. Potentially explosive reaction with aluminum chloride + nitromethane (at 1 10°C/lOO bar), formaldehyde, perijxydisulfuric acid, peroxymonosulfuric acid, sodium nitrite + heat. Violent reaction with aluminum chloride + nitrobenzene (at 120℃), sodium nitrate + trifluoroacetic acid, butadiene. Can react with oxidizing materials. To fight fire, use alcohol foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes. | [Hazardous Substances Data]
108-95-2(Hazardous Substances Data) | [IDLA]
250 ppm |
| Questions And Answer | Back Directory | [Chemical Properties]
Phenol is the simplest member of a class of organic compounds possessing a hydroxyl group attached to a benzene ring or to a more complex aromatic ring system.
Also known as carbolic acid or monohydroxybenzene, phenol is a colorless to white crystalline material of sweet odor, having the composition C6H5OH, obtained from the distillation of coal tar and as a by-product of coke ovens.
Phenol has broad biocidal properties, and dilute aqueous solutions have long been used as an antiseptic. At higher concentrations, it causes severe skin burns; it is a violent systemic poison. It is a valuable chemical raw material for the production of plastics, dyes, pharmaceuticals, syntans, and other products.

Phenol melts at about 43°C and boils at 183°C. The pure grades have melting point of 39°C, 39.5°C, and 40°C. The technical grades contain 82%-84% and 90%-92% phenol. The crystallization point is given as 40.41°C. The specific gravity is 1.066. It dissolves in most organic solvents. By melting the crystals and adding water, liquid phenol is produced, which remains liquid at ordinary temperatures. Phenol has the unusual property of penetrating living tissues and forming a valuable antiseptic. It is also used industrially in cutting oils and compounds and in tanneries. The value of other disinfectants and antiseptics is usually measured by comparison with phenol. | [Uses]
Phenol is an important organic chemical raw material, widely used in the production of phenolic resin and bisphenol A, in which bisphenol A is important raw material for polycarbonate, epoxy resin, polysulfone resin and other plastics. In some cases the phenol is used to produce iso-octylphenol, isononylphenol, or isododecylphenol through addition reaction with long-chain olefins such as diisobutylene, tripropylene, tetra-polypropylene and the like, which are used in production of nonionic surfactants. In addition, it can also be used as an important raw material for caprolactam, adipic acid, dyes, medicines, pesticides and plastic additives and rubber auxiliaries.
| [Production]
Coal tar was once the main source of phenol, and was extracted from sodium hydroxide solution. In earlier time, people use sulfonation method to produce phenol: react sodium benzene sulfonate with sodium hydroxide to generate the sodium salt of phenol, and then treat it with acid to obtain phenol. In recent years, hydrolyzing chlorobenzene or oxidizing cumene has become the major production method. The by-product acetone in latter method is also an important industrial raw material, so oxidizing cumene is more economic industrially thus widely applied.
Cumene method:
This method generates cumene from propylene and benzene in the presence of aluminum trichloride. It oxidizes to cumene hydroperoxide and then decomposes with cation exchange resin to give phenol and acetone. For each ton of phenol produced, 0.62 tons of acetone can be produced.
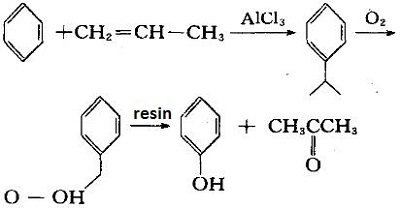
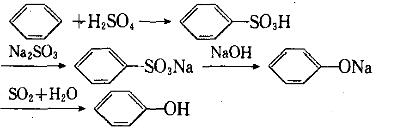
Sulfonation method:
se sulfuric acid to sulfonate benzene to generate benzene sulfonic acid, neutralize it with sodium sulfite, and then undergo acidification and vacuum distillation in caustic soda solution.
Hydrogen benzene hydrolysis method: hydrogen benzene is hydrolyzed in caustic soda solution with high temperature and high pressure to generate phenol sodium, which is then neutralized to give phenol. | [Toxicity]
Phenol is highly corrosive and toxic. It mainly affects the central nervous system. The oral lethal dose for adults is 1 g. It can be irritating, numbing or necrotizing to the skin. It is toxic to skin contact, swallowing or inhalation of phenol. Misuse of a small amount of phenol can cause nausea, vomiting, shock, coma and even death in case of respiratory failure. Very few amounts are used as a preservative, so that adverse reactions are rarely found.
Due to its high toxicity, it has been replaced by more effective and less toxic phenolic derivatives.
|
|
|