| Identification | More | [Name]
2,4-Pentanedione | [CAS]
123-54-6 | [Synonyms]
2,3-PENTANEDIONE
2,4-PENTADIONE
2,4-PENTANEDIONE
ACAC
ACETYLACETONE
Acetylaeetone
ACETYLPROPIONYL
AKOS BBS-00004242
DIACETYLMETHANE
FEMA 2841
METHYL ETHYL DIKETONE
PENTAN-2,3-DIONE
PENTANE-2.3-DIONE
TIMTEC-BB SBB009914
2,4-Diketopentane
2,4-Dioxopentane
2,4-Pentandione
2-Propanone, acetyl-
Acetoacetone
Acetone, acetyl- | [EINECS(EC#)]
209-984-8 | [Molecular Formula]
C5H8O2 | [MDL Number]
MFCD00008787 | [Molecular Weight]
100.12 | [MOL File]
123-54-6.mol |
| Chemical Properties | Back Directory | [Description]
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid
odour. It is readily soluble in water and in organic solvents and incompatible with light,
ignition sources, excess heat, oxidising agents, strong reducing agents, and strong bases. On decomposition, acetylacetone releases hazardous products such as carbon monoxide,
irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the
production of anti-corrosion agents and its peroxide compounds for the radical initiator
application for polymerisation. It is used as a chemical intermediate for drugs (such as
sulphamethazine, nicarbazine, vitamin B6, and vitamin K) and pesticides sulfonylurea
herbicides and pesticides. It is used as an indicator for the complexometric titration of Fe
(III), for the modification of guanidino groups and amino groups in proteins, and for the
preparation of metal acetylacetonates for catalyst application. | [Appearance]
2,4-Pentanedione is a colorless to yellowish liquid with a sour, rancid odor. The Odor Threshold is 0.01 ppm. | [Melting point ]
-23 °C (lit.) | [Boiling point ]
140.4 °C (lit.) | [density ]
0.975 g/mL at 25 °C(lit.)
| [vapor density ]
3.5 (vs air)
| [vapor pressure ]
6 mm Hg ( 20 °C)
| [refractive index ]
n20/D 1.452(lit.)
| [Fp ]
66 °F
| [storage temp. ]
2-8°C
| [solubility ]
H2O: soluble1 in 8 parts | [form ]
Liquid | [pka]
8.9(at 25℃) | [color ]
very deep green-yellow
| [Odor]
pleasant odor | [PH]
6 (200g/l, H2O, 20℃) | [Relative polarity]
0.571 | [explosive limit]
2.4-11.4%(V) | [Water Solubility ]
16 g/100 mL (20 ºC) | [Merck ]
14,81 | [BRN ]
741937 | [Dielectric constant]
23.1(20℃) | [Exposure limits]
No exposure limit has been set. | [Cosmetics Ingredients Functions]
ADHESIVE | [InChIKey]
YRKCREAYFQTBPV-UHFFFAOYSA-N | [LogP]
0.68 at 20℃ | [CAS DataBase Reference]
123-54-6(CAS DataBase Reference) | [NIST Chemistry Reference]
Acetylacetone(123-54-6) | [EPA Substance Registry System]
123-54-6(EPA Substance) |
| Hazard Information | Back Directory | [Chemical Properties]
Acetylacetone (2,4-pentanedione) is a clear or slightly yellowish liquid with a putrid
odor. It is readily soluble in water. It is with other incompatible materials, light, ignition
sources, excess heat, oxidizing agents, strong reducing agents, and strong bases. On
decomposition, acetylacetone releases hazardous products, such as carbon monoxide,
irritating and toxic fumes and gases, and carbon dioxide. Acetylacetone is used in the
production of anticorrosion agents and its peroxide compounds for the radical initiator
application for polymerization. It is used as a chemical intermediate for drugs (such as
sulfamethazine, nicarbazine, vitamin B6, and vitamin K), sulfonylurea herbicides, and
pesticides. It is used as a solvent for cellulose acetate, as an additive in gasoline and
lubricant, as a dryer of paint and varnish. It is used as an indicator for the complexometric
titration of Fe(III), for the modifi cation of guanidino groups and amino groups in
proteins, and in the preparation of metal acetylacetonates for catalyst application. | [General Description]
A colorless or yellow colored liquid. Less dense than water. Flash point 105°F. Vapors are heavier than air. Used as a solvent in paints and varnishes. | [Reactivity Profile]
Ketones, such as 2,4-PENTANEDIONE(123-54-6), are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. May dissolve plastics [USCG, 1999]. | [Air & Water Reactions]
Flammable. Soluble in water. | [Health Hazard]
Exposures to acetyl acetone cause eye irritation, chemical conjunctivitis, corneal damage,
and skin irritation (harmful if absorbed through the skin). At low concentrations for long
periods, inhalation/dermal absorption of acetyl acetone causes irritation and dermatitis,
cyanosis of the extremities, pulmonary edema, and a burning sensation in the chest.
Ingestion/accidental ingestion in the workplace can result in gastrointestinal irritation,
nausea, vomiting, diarrhea, and CNS depression. Inhalation of high concentrations may
cause CNS effects characterized by nausea, headache, dizziness or suffocation, unconsciousness,
and coma. The target organ of acetyl acetone poisoning has been identifi ed as
the CNS. | [Health Hazard]
Inhalation causes dizziness, headache, nausea, vomiting and loss of consciousness. Contact with liquid irritates eyes. | [Potential Exposure]
Acetoacetic acid derivative. 2,4-Pentanedione is used in gasoline and lubricant additives, fungicides, insecticides, and colors manufacture; as a chemical intermediate and in the manufacture of metal chelates | [Fire Hazard]
Behavior in Fire: Vapor is heavier than air and may travel to a source of ignition and flash back. | [First aid]
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. | [Shipping]
UN2310 Pentane-2,4-dione, Hazard Class: 3; Labels: 3-Flammable liquid | [Incompatibilities]
Vapors may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. reducing agents; halogens, aliphatic amines; alkanolamines, organic acids; isocyanates. Strong light may cause polymerization. | [Waste Disposal]
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. | [Application]
Acetylacetone, also known as 2,4-pentanedione, is an important commodity chemical and widely used as a fuel additive, as dyeing intermediate, in the fields of metal extraction, metal plating, and resin modification. Hantzsch reaction was used as a derivatizing agent for the assay of compounds having a primary amino group. The reagent was reacted with the primary amino group of the drugs to form a product having color and/or emit fluorescence. This condensation reaction was distinguished by its precision, reproducibility, and analytical cost reduction. FLX contains an aliphatic amino group, in the presence of formaldehyde solution, this amino group can condense with two equivalents of acetylacetone to form dihydropyridine derivative that emits yellow fluorescent product. (Figure1). Under optimized conditions of the reaction, FLX gave highly fluorescent product measured at λem 479 nm using 419 nm as excitation. | [Definition]
ChEBI: A beta-diketone that is pentane in which the hydrogens at positions 2 and 4 are replaced by oxo groups. | [Production Methods]
2,4-Pentanedione is produced by thermal or metal-catalyzed rearrangement of isopropenyl acetate(obtained from acetone and ketene):
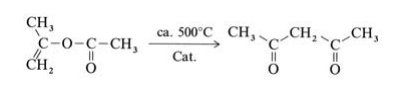
Isopropenyl acetate vapor is fed at atmospheric pressure through a V2A steel tube with an inner temperature of 520℃. The hot reaction gases are quenched, condensed, and cooled to 20℃, whereby the gaseous byproducts carbon monoxide, carbon dioxide, methane, and ketene are separated. The product is purified by fractional distillation. Other industrially less important processes for the production of 2,4-pentanedione, include the Claisen ester condensation of ethyl acetate with acetone using sodium ethoxide as condensation agent and the acetylation of acetoacetic acid esters with acetic anhydride in the presence of magnesium salts.
| [Synthesis Reference(s)]
Journal of the American Chemical Society, 102, p. 2095, 1980 DOI: 10.1021/ja00526a059
Organic Syntheses, Coll. Vol. 3, p. 17, 1955 | [Purification Methods]
Small amounts of acetic acid are removed by shaking with small portions of 2M NaOH until the aqueous phase remains faintly alkaline. The sample, after washing with water, is dried with anhydrous Na2SO4, and distilled through a modified Vigreux column (p 11) Cartledge J Am Chem Soc 73 4416 1951]. An additional purification step is fractional crystallisation from the liquid. Alternatively, there is less loss of acetylacetone if it is dissolved in four volumes of *benzene and the solution is shaken three times with an equal volume of distilled water (to extract acetic acid): the *benzene is then removed by distillation at 43-53o and 20-30mm through a helices-packed column. It is then refluxed over P2O5 (10g/L) and fractionally distilled under reduced pressure. The distillate (sp conductivity 4 x 10-8 ohm-1cm-1) is suitable for polarography [Fujinaga & Lee Talanta 24 395 1977]. To recover used acetylacetone, metal ions are stripped from the solution at pH 1 (using 100mL 0.1M H2SO4/L of acetylacetone). The acetylacetone is then washed with (1:10) ammonia solution (100mL/L) and with distilled water (100mL/L, twice), then treated as above. It complexes with Al, Be, Ca, Cd, Ce , Cu, Fe2+, Fe3+ , Mn, Mg, Ni, Pb and Zn. [Beilstein 1 H 777, 1 I 401, 1 II 831, 1 III 3113, 1 IV 3662.] | [Toxics Screening Level]
The Initial Threshold Screening Level (ITSL) for 2,4-pentanedione is 25 μg/m3 with
annual averaging time. |
| Safety Data | Back Directory | [Hazard Codes ]
Xn,Xi | [Risk Statements ]
R11:Highly Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin .
R22:Harmful if swallowed.
R10:Flammable. | [Safety Statements ]
S21:When using, do not smoke .
S23:Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer) .
S24/25:Avoid contact with skin and eyes .
S36:Wear suitable protective clothing .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
UN 2310 3/PG 3
| [WGK Germany ]
1
| [RTECS ]
SA1925000
| [F ]
9-23 | [Autoignition Temperature]
662 °F | [TSCA ]
Yes | [HazardClass ]
3 | [PackingGroup ]
III | [HS Code ]
29141900 | [storage]
Acetylacetone should be stored away from heat, sparks, flame, and from sources of ignition.
It should be stored in a tightly sealed container, in a cool, dry, well-ventilated area,
away from incompatible substances. | [Precautions]
Occupational workers should only use/handle acetyl acetone in a well-ventilated area,
with spark-proof tools and explosion-proof equipment. Workers should not cut, weld,
braze, solder, drill, grind, pressurize, or expose empty containers to heat, sparks, or
flames. | [Safety Profile]
Poison by ingestion and intraperitoneal routes. Moderately toxic by inhalation. A skin and severe eye irritant. Experimental reproductive effects. Mutation data reported. Flammable liquid when exposed to heat or flame. Incompatible with oxidning materials. To fight fire, use alcohol foam, CO2, dry chemical. | [Hazardous Substances Data]
123-54-6(Hazardous Substances Data) | [Toxicity]
LC50 (4 hrs) in rats: 1000 ppm (Carpenter) |
| Raw materials And Preparation Products | Back Directory | [Raw materials]
Acetic acid-->Sodium-->Acetone-->Carbon disulfide-->4-Chlorobenzaldehyde-->Sodium amide-->Copper (II) acetate monohydrate-->ketene-->Triethyl phosphate-->Isopropenyl acetate-->Acetovanillone | [Preparation Products]
Tetrahydrocurcumin-->1,2-DIHYDRO-4-(METHOXYMETHYL)-6-METHYL-5-NITRO-2-OXONICOTINONITRILE-->4-methoxymethylpyridoxine-->4,6-DIMETHYL-PYRIMIDINE-2-SULFONYL FLUORIDE-->5-BROMO-4,6-DIMETHYL-1H-PYRAZOLO[3,4-B]PYRIDIN-3-AMINE-->2-CHLORO-3-(3,5-DIMETHYL-PYRAZOL-1-YL)-QUINOXALINE-->4,6-DIMETHYL-2-HYDROXYPYRIMIDINE HYDROCHLORIDE-->1-(4-METHYL-2-(METHYLTHIO)PYRIMIDIN-5-YL)ETHANONE-->2-(CARBOXYMETHYLTHIO)-4,6-DIMETHYLPYRIMIDINE MONOHYDRATE-->Ninhydrin hydrate-->5-AMINO-4-(METHOXYMETHYL)-6-METHYL-3-PYRIDINEMETHANAMINE-->5-BROMO-2-CHLORO-4,6-DIMETHYLNICOTINONITRILE-->2-(2-CARBOXYETHYL)THIO-4,6-DIMETHYLPYRIMIDINE-->17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal-->2-AMINO-4,6-DIMETHYL-3-PYRIDINECARBOXAMIDE-->3-(PYRIMIDIN-2-YLTHIO)PENTANE-2,4-DIONE-->3-(3,5-DIMETHYL-PYRAZOL-1-YL)-PROPYLAMINE-->1,4,6-Trimethyl-1H-pyrazolo[3,4-b]pyridin-3-ylamine ,97%-->4,6-DIMETHYL-1H-PYRAZOLO[3,4-B]PYRIDIN-3-AMINE-->Bis(2,4-pentanedionato-O,O')palladium(II)-->2-Chloro-3-cyano-4,6-dimethylpyridine-->4,6-Dimethyl-2-hydroxypyridine-->5-CYANO-6-HYDROXY-4-METHOXYMETHYL-2-METHYLPYRIDINE-->2-(4-Aminobenzenesulfonamido)-4,6-dimethylpyrimidine-->17-Ethinyl-3,17-dihydroxy-18-methylestra-2,5(10)-diene3-methylether-->6-CHLORO-5-CYANO-4-METHOXYMETHYL-3-NITRO-2-PICOLINE-->4,6-Dimethyl-2-methylmercapyrimidine-->1-(2,4-DIMETHYLQUINOLIN-3-YL)ETHANONE HYDROCHLORIDE-->3-Cyano-4,6-dimethyl-2-hydroxypyridine-->3-ACETYL-2-METHYL-QUINOLINE-4-CARBOXYLIC ACID-->3,5-DIMETHYL-1H-PYRAZOLE-4-CARBOXYLIC ACID-->3,5-DIMETHYLPYRAZOLE-1-CARBOXAMIDINE NITRATE-->4,6-DIMETHYL-2-THIOPYRIMIDINE-->MEQUINDOX-->17-Ethynyl-18-methylestra-5(10),9(11)-dien-17-ol-3-one-->2-Ethyl-3-methylpyrazine-->2-Amino-4,6-dimethylpyrimidine |
| Questions And Answer | Back Directory | [Acetone derivatives]
Acetylacetone is a derivative of acetone; chemical formula: CH3COCH2COCH3; It is colorless to pale yellow transparent liquid. It is usually the mixture is enol form and keto form which are the tautomers of each other; these two forms are in dynamic equilibrium; enol isomer forms hydrogen bonds inside the molecule; in the mixture, the keto form accounts for about 18% and enol type accounted for 82%. Cool the petroleum ether of their mixture to-78 °C so the enol will be precipitated as a solid and the two forms will be separated with each other. Upon the enol form being returned back to room temperature, the above equilibrium will be restored.
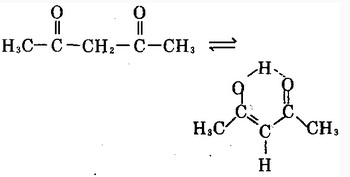
Acetylacetonate have a pleasant odor. It is flammable, and has a relative molecular mass of 100.13. Its relative density is 0.9721 (25 °C). Its melting point is-23.5 °C and boiling point is 140.5 °C or 139 °C (99.458 × 103Pa). Its flash point is 41 °C. The refractive index is 1.4494. It has a vapor pressure of 0.800 × 103Pa (20 °C). (At 20 °C 16.9,80 °C at 34) and is soluble in water, ethanol, benzene, chloroform, ether, acetone, ethyl acetate and acetic acid. It is susceptible to hydrolysis to generate acetic acid and acetone. The molecular structure of the acetylacetone is a saturated diketone structure in which two hydroxyl groups are connected by a methylene group; this form is usually referred to as β-diketone. Acetylacetone is also one of the simplest saturated β-diketones and is a derivative of acetone. It has an active chemical property and can react with ferric chloride aqueous solution to exhibit a dark red color. This product can almost react with the hydroxides, carbonates or acetates of all metals to form a complex with a general formula being (C5H7O2) • M, wherein M corresponds to the metal element and n is the metal compounds. Most of such compounds are stable, and many of them are soluble in many organic solvents. This product can have reaction with chlorine in the presence of light of with only two ends of methyl hydrogen being replaced by chlorine. When this product is reacted with sodium, it can release hydrogen and generates sodium acetylacetonate. Acetylacetonate have a narcotic effect and can stimulate the skin and mucous membranes; at high concentrations (100 × 10-6 or more), it is easy to produce some symptoms of poisoning such as nausea, headache, and dizziness. Rat oral LD50: 970mg/kg.
| [Intermediates of organic synthesis]
Acetylacetone is an important intermediate for organic synthesis which is widely used in pharmaceutical, perfume, pesticides and other industries.
Acetylacetone is an important raw material in the pharmaceutical industry, such as for the synthesis of 4,6-dimethyl-pyrimidine derivatives. It can also be used as the solvents for cellulose acetate, the drying agent for paints and varnishes, etc., and are also important analytical reagents.
Due to the presence of enol, acetylacetone can form chelate with a variety of metals such as cobalt (II), Co (III), beryllium, aluminum, and chromium, iron (II), copper, nickel, palladium, zinc, indium, tin, zirconium, magnesium, manganese, scandium and thorium; it can also be used as fuel additives and lubricant additives.
Taking advantage of its chelation reaction with many kinds of metals, it can be used as a kind of metal cleaning agent for micropore; It can also used as a catalyst, a resin cross-linking agent, the resin curing accelerator; resins, rubber additives; for the hydroxylation reaction, hydrogenation reaction, isomerized reaction, and the synthesis of low molecular weight unsaturated ketone as well as polymerization and copolymerization of low-carbon olefins; it can also be used as an organic solvent for dissolving cellulose acetate, ink, and paint; it can also used as paint drying agent; it can also be used as the raw materials for preparation of insecticide, fungicide materials, and animals laxatives as well as feed additives; it can also be used as infrared reflective glass, a transparent conductive film (indium salt), a superconducting thin film (indium salt) forming agent; acetylacetone metal complexes has special colors (green copper salts, iron red, purple chromium salt) and is insoluble in water; it can also be used as pharmaceutical raw materials and raw materials for organic synthesis.
The above information is edited by the chemicalbook of Dai Xiongfeng.
| [Chemical Properties]
This product is colorless or slightly yellow transparent liquid with an unpleasant odor, m.p.-23 °C, bp140.4 °C, n20D:1.4520, the relative density is 0.975, miscible with alcohol, ether, chloroform, acetone, acetic acid and some other organic solvents; it is also soluble in water; this product is flammable and corrosive.
| [Uses]
Pentanedione, also known as Acetylacetone, is the intermediates of fungicides such as methyl mepanipyrim, mepanipyrim and herbicide pyrazosulfuron-methyl. It can be used as a analysis reagent and the aluminum extraction agent from tungsten, molybdenum. Acetylacetone is a kind of intermediate of organic synthesis which produces amino-4,6-dimethyl-pyrimidine with guanidine; it is an important pharmaceutical raw materials. It can be used as the solvent for cellulose acetate, as gasoline and lubricant additives, as desiccants of paints and varnishes, and as fungicides as well as insecticides. Acetylacetone can also serve as a catalyst for cracking petroleum, hydrogenation and hydroformylation reactions as well as being the oxidation promoting agent of oxygen. It can be used to remove the metal oxides in porous solid and used for processing polypropylene. In the United States and Europe, it is used for the antidiarrheal medicine and livestock feed additives in more than 50% of cases. | [Toxicity]
Moderate toxicity, can stimulate skin and mucous membrane. If the human body stays at 150 ~ 300mg/kg for a long time, it will have symptoms such as headache, nausea, vomiting, vertigo and sensory retardation. | [Category]
Flammable liquids
| [Toxicity grading]
Poisoning
| [Acute toxicity]
Oral-rat LD50: 55 mg/kg; Oral-Mouse LD50: 951 mg/kg
| [Stimulus data]
Skin-rabbit 488 mg with mild effect; Eyes-rabbit 20 mg with mild effect;
| [Flammability and hazard characteristics]
Easily flammable in case of fire, heat, and oxidants with burning producing irritating smoke irritation
| [Storage Characteristics]
Treasury: ventilation, low-temperature and dry; store separately from oxidants
| [Extinguishing agent]
Dry, dry sand, carbon dioxide, foam, 1211 fire extinguishing agent
|
|
|