| Identification | More | [Name]
Prulifloxacin | [CAS]
123447-62-1 | [Synonyms]
PRULIFLOXACIN
1h,4h-(1,3)thiazeto(3,2-a)quinoline-3-carboxylicacid,6-fluoro-1-methyl-7-(4-(
3-dioxol-4-yl)methyl)-1-piperazinyl)-4-oxo-(5-methyl-2-oxo-
nm441
SEA BUCKTHORN P.E 20%
Prulifloxacine
PRULIFLOXACIN(FORRESEARCHONLY)
(+/-)-7-{4-[(Z)-2,3-Dihydroxy-2-butenyl]-1-piperazinyl}-6-fluoro-1-methyl-4-oxo-1H,4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid cyclic carbonate
6-Fluoro-1-methyl-7-(4-(5-methyl-2-oxo-1,3-dioxelen-4-yl)methyl-1-piperazinyl)-4-oxo-4H-(1,3)thiazeto(3,2-a)quinoline-3-carboxylic acid
(+/-)-7-{4-[(Z)-2,3-Dihydroxy-2-butenyl]-1-piperazinyl}-6-fluoro-1-methyl-4-oxo-1H,4H-[1,3]thiazeto[3,2-a]quinoline-3-carboxylic acid cyclic carbonate | [EINECS(EC#)]
819-932-1 | [Molecular Formula]
C21H20FN3O6S | [MDL Number]
MFCD00864847 | [Molecular Weight]
461.46 | [MOL File]
123447-62-1.mol |
| Chemical Properties | Back Directory | [Melting point ]
211-214°C | [Boiling point ]
633.2±65.0 °C(Predicted) | [density ]
1.62±0.1 g/cm3(Predicted) | [storage temp. ]
Sealed in dry,Store in freezer, under -20°C | [solubility ]
1 M NaOH: soluble25ML, clear, colorless (Solvent: 1 mg + 25 mL of NaOH) | [form ]
White to yellow crystalline solid. | [pka]
5.85±0.40(Predicted) | [color ]
Off-White to Pale Yellow | [λmax]
276nm(H2O)(lit.) | [Merck ]
14,7908 | [InChI]
InChI=1S/C21H20FN3O6S/c1-10-16(31-21(29)30-10)9-23-3-5-24(6-4-23)15-8-14-12(7-13(15)22)18(26)17(20(27)28)19-25(14)11(2)32-19/h7-8,11H,3-6,9H2,1-2H3,(H,27,28) | [InChIKey]
PWNMXPDKBYZCOO-UHFFFAOYSA-N | [SMILES]
N12C(C)SC1=C(C(O)=O)C(=O)C1=C2C=C(N2CCN(CC3=C(C)OC(=O)O3)CC2)C(F)=C1 | [CAS DataBase Reference]
123447-62-1(CAS DataBase Reference) |
| Safety Data | Back Directory | [WGK Germany ]
WGK 3 | [RTECS ]
XJ0600000 | [HS Code ]
2941.90.3000 | [Storage Class]
11 - Combustible Solids | [Hazard Classifications]
Lact.
Skin Irrit. 2
STOT RE 2 |
| Questions And Answer | Back Directory | [Abstract]
Prulifloxacin, a prodrug of Ulifloxacin inhibits replication of bacterial DNA (nuclear material) by inhibition of DNA-gyrase in microbes, is a newer class of fluoroquinolone exerts broad-spectrum anti-bacterial activity against several Gram-positive and Gram-negative organisms. Prulifloxacin is generally prescribed for the treatment of uncomplicated lower urinary tract infections, acute exacerbations of chronic bronchitis and complicated lower urinary tract infections. | [Quinolone antibiotics]
Prulifloxacin is a quinolone antibiotic, a fluoroquinolone antibiotics prodrug NM394 by the Japan Pharmaceutical Co. and Meiji Seika Kaisha, Ltd. joint research and development in the late eighties, and it was first approved for marketing in December 2002 in Japan. the dosage form is 100mg tablets, by inhibiting bacterial DNA topoisomerase ⅱ and ⅳ activity and bacterial DNA synthesis, completing sterilization, which is different from the traditional model, without drug resistance to other classes of antibiotics. It is used for the treatment of respiratory gram-positive bacteria and negative bacteria clinically, which is caused by infections, urogenital infections, otolaryngology infection, biliary tract infections, enteritis, skin and soft tissue infections and surgical infections. Notable features are as follows:
- Anti-gram-positive bacteria are similar with ciprofloxacin. The anti-Gram-negative bacteria, led by pseudomonas aeruginosa, is better than the similar products, such as ciprofloxacin, ofloxacin, levofloxacin, resistance to green pus bacillus particularly high sensitivity, and the antibacterial abilitu is better than the gatifloxacin class and other antibiotics currently listed.
- The oral absorption is good without savings and cerebrospinal fluid distribution, and the side effects is little.
- The half-life is as long as 8-9 hours, and the number of eating medication is few.
- Rapid onset, time to peak within 0.5 to 1 hour, the effet is stronger (due to bacterial accumulation in good).
| [Indication]
It is used for the treatment of prulifloxacin sensitive staphylococcus, streptococcus, Neisseria gonorrhoeae, (except typhoid, paratyphoid) pneumoniae, Enterococcus, Moraxella Alex Salmonella, E. coli, Shigella, Salmonella, lemon acid bacteria, Klebsiella pneumoniae, Serratia, Proteus, Vibrio cholerae, Pseudomonas aeruginosa, Streptococcus digestion, they can cause the following infections:
- Superficial skin infections (acute superficial folliculitis, infectious impetigo), deep skin infections (cellulitis, erysipelas, boils, boils swollen disease, appendicitis, suppurative paronychia), chronic pyoderma (sebaceous cyst infection, purulent, etc.).
- Superficial secondary infections, such as abscess, trauma, burns, and surgical trauma.
- Acute respiratory infections (pharyngitis, tonsillitis, acute bronchitis), chronic respiratory disease secondary infection (chronic bronchitis, diffuse bronchiolitis, bronchiectasis, emphysema, pulmonary fibrosis, bronchial asthma, etc.) and pneumonia.
- Cystitis, pyelonephritis, prostatitis.
- Cholecystitis, cholangitis.
- Infectious enteritis, bacillary dysentery, salmonellosis, cholera.
- The uterine infection, adnexitis.
- Blepharitis, stye.
- Otitis media, sinusitis.
| [Pharmacokinetics]
After oral administration, Prulifloxacin is rapidly metabolized as an active form of drug, Ulifloxacin. The drug exerts good penetration into the target tissue with prolonged elimination half-life with once-daily administration.
After absorption, Cmax is generally achieved within one hour, and apparent Vss is 1231 L. About 45% of the administered drug is bound to plasma proteins. The drug is extensively metabolized by first-pass metabolism into active metabolites. The terminal half-life of Ulifloxacin is 10.6-12.1 hours. The drug is mainly excreted via the urine as unmetabolized drug. | [Prulifloxacin Intermediate]
Prulifloxacin Intermediate PL-7
Appearance: white to light yellow powder
Chemical name: 6,7-difluoro-2-mercapto-ethyl-4-hydroxy-quinoline-3-carboxylic acid ethyl ester CAS: 154330-68-4
Molecular Formula: C14H13F2NO3S
Prulifloxacin intermediate PL-9
Appearance: white to light yellow powder
Chemical name: 6,7-difluoro-1-methyl-4-oxo--4H-(1,3) thiazine (3,2a) and quinoline-3-carboxylate CAS: 113046-72-
Molecular Formula:C14H11F2NO3S
Prulifloxacin Intermediate PL-10
Appearance: white to light yellow powder
Chemical Name: 6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl) 4H-(1,3) thiazine (3,2a) and quinoline-3-carboxylate CAS: 113028-17-4
Molecular Formula:C18H20FN3O3S
Prulifloxacin Intermediate PL-11
Appearance: white to light yellow powder
Chemical Name: 6-fluoro-1-methyl-4-oxo-7-(1-piperazinyl)-4H-(1,3) thiazine (3,2a) and quinoline-3-carboxylic acid CAS : 112984-60-8
Molecular Formula:C16H16FN3O3S
Application:For the total synthesis of gatifloxacin antibacterials | [Side effects]
Abdominal cramp, Confusion, Diarrhoea, Drowsiness, Altered taste, Epigastric pain, Gastritis, Headache, Hot flashes, Increased bilirubin in the blood, Itching, Loss of appetite, Sedation, Skin rash, Sleep disorder, Vomiting, Nausea. | [References]
https://www.ncbi.nlm.nih.gov/pubmed/15456336
http://www.drugsupdate.com/generic/view/1110/Prulifloxacin |
| Hazard Information | Back Directory | [Description]
Prulifloxacin was launched as the third fluoroquinone. It was introduced in
Japan as an oral treatment for urinary tract infections (UTls), respiratory tract infections
(RTls) and bacterial pneumoniae. It can be synthesized in 10 steps from commercially
available 3,4-difluoroaniline. Key steps involve the cyclization of 6,7-difluoro-rl-hydroxy-2-
thioquinoline-3carboxylic acid ethyl ester with 1 ,I-dibromomethane to give the
corresponding thiazeto-[3,2a]quinoline. Aromatic nucleophilic substitution of the 7-fluoro
atom with piperazine followed by hydrolysis of the ethyl ester and finally alkylation of the
piperazinyl moiety with 4-(bromomethyl)-5-methyl-l ,bdioxol-Bone complete the synthesis.
Prulifloxacin is a lipophilic prodrug, which is rapidly hydrolyzed to the corresponding Ndealkylated
piperazine, NM 394, by paraoxonase type enzymes in blood and liver following
intestinal absorption. The DNA gyrase inhibitor NM 394 accounts for all antimicrobial
activity: it shows a similar or greater activity against gram-positive bacteria compared to
ciprofloxacin, and a greater activity in the case of gram-negative bacteria. In clinical
studies, prulifloxacin has shown good efficacy against UTls and RTls. The drug is mainly
excreted in the urine and in the feces as unchanged NM 394, which has a plasma half-life
of approximately 8 h. Phototoxicity in animal models is less severe than with other
quinolones. Prulifloxacin is well tolerated with an adverse effect profile similar to that of
other fluoroquinolones. | [Chemical Properties]
Off-White Solid | [Originator]
Nippon Shinyaku (Japan) | [Uses]
Fluoroquinoline antibacterial; prodrug for active metabolite, Ulifloxacin. Antibacterial. | [Uses]
Prulifloxacin is a synthetic chemotherapeutic antibiotic of the fluoroquinolone drug class. Prulifloxacin is a prodrug for active metabolite, Ulifloxacin. Antibacterial. | [Definition]
ChEBI: Prulifloxacin is a quinolone antibiotic and a fluoroquinolone antibiotic. | [Brand name]
Sword | [Pharmaceutical Applications]
A lipophilic prodrug which is very rapidly metabolized by esterase into ulifloxacin, a 6-fluoro, 7-piperazinyl thiazetoquinoline.
Ulifloxacin is moderately active against Staph. aureus (MIC 0.4–0.8 mg/L) and inactive against Str. pneumoniae (MIC 2–8 mg/L) as well as against Enterococcus spp. Against Enterobacteriaceae (MIC 0.05–0.8 mg/L) and Ps. aeruginosa (MIC 0.2–0.8 mg/L) activity is similar to that of ciprofloxacin. It is active against fastidious Gram-negative bacilli, but not against anaerobes and non-fermentative Gram-negative bacilli. Activity against Acinetobacter spp. is modest.
Prulifloxacin is rapidly converted into ulifloxacin and after 3 h is no longer detected in blood. In volunteers receiving a single oral dose, peak plasma concentrations of 0.68 mg/L (300 mg dose) to 1.88 mg/L (for 400 mg dose) were attained between 0.67 and 1.25 h. The mean apparent elimination half-life was 8 h and the mean cumulative elimination rate in urine within 48 h was 31–46%. Other inactive metabolites account for 7% of the dose. Half the administered dose is eliminated in feces within 72 h as ulifloxacin and 4% as prulifloxacin. Protein binding is 45%. | [Synthesis]
The synthesis of
prulifloxacin (22)started with the treatment of 3,4-
difluoroaniline (183) with carbon disulfide in the presence of
TEA to give the triethylammonium dithiocarbamate, which
by reaction with ethyl chloroformate and TEA in
chloroform, was converted into isothiocyanate 184 in 74%
yield. Reaction of 184 with diethyl malonate in the presence
of KOH in dioxane yielded methylenemalonate 185
potassium salt, which was ethylated with ethyl sulfate in
ethanol to give compound 186 in excellent yield. 6,7-
Difluoroquinoline 187 was obtained with the highest yield
and regioselectivity when precursor 186 was heated in
refluxing xylene. To suppress the side reaction in the
subsequent chlorination, quinoline 187 was acylated to give
188 with acetyl chloride in chloroform. Chlorination of 188
with sulfuryl chloride gave compound 189 in 79% yield.
Compound 189 was treated with sodium acetate in THF to
afford cyclized compound 190, which was condensed with
piperazine in DMF to give compound 191. The hydrolysis
of ester 191 with KOH in hot t-butanol gave free acid 192,
which was finally condensed with 4-(bromomethyl)-5-
methyl-1, 3-dioxol-2-one (163) by treatment of potassium
bicarbonate in DMF to give prulifloxacin (22).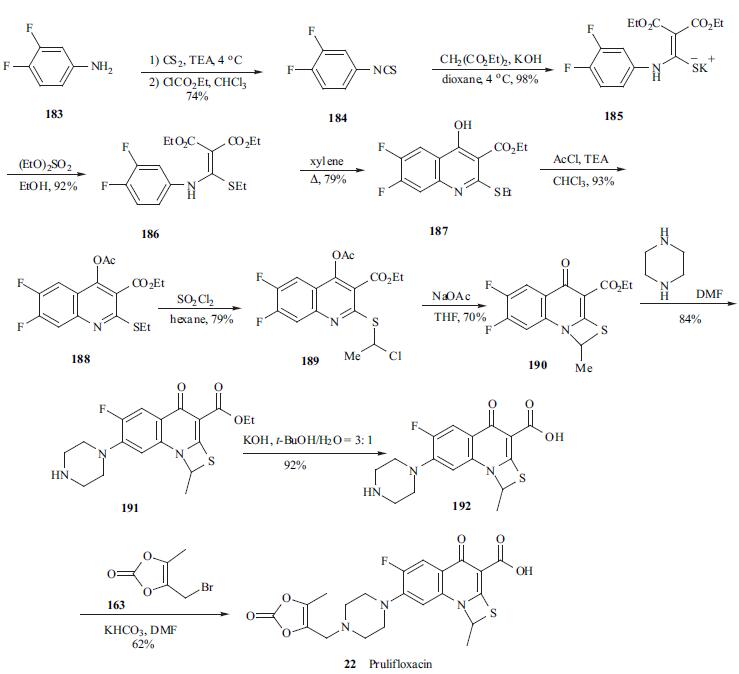
|
|
|