| Identification | More | [Name]
Norfloxacin | [CAS]
70458-96-7 | [Synonyms]
1-ETHYL-6-FLUORO-1,4-DIHYDRO-4-OXO-7-(1-PIPERAZINLY)-3-QUINOLINECARBOXYLIC ACID
1-ETHYL-6-FLUORO-1,4-DIHYDRO-4-OXO-7-(1-PIPERAZINYL)-3-QUINOLINECARBOXYLIC ACID
1-ETHYL-6-FLUORO-1,4-DIHYDRO-4-OXO-7-PIPERAZINO-3-QUINOLINECARBOXYLIC ACID
1-ETHYL-6-FLUORO-4-OXO-7-PIPERAZIN-1-YL-1,4-DIHYDRO-QUINOLINE-3-CARBOXYLIC ACID
AKOS NCG-0042
baccidal
BACCIDAL BARAZAN
chibroxin
CHIBROXINE
floxacin 400
MK-366
n-desmethylpefloxacin
NORFLOXACIN
NORFLOXACINE
NORFLOXACIN LACTATE
noroxin
norphloxacine
1,4-dihydro-1-ethyl-6-fluoro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylica
1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylica
am-715 | [EINECS(EC#)]
274-614-4 | [Molecular Formula]
C19H24FN3O6 | [MDL Number]
MFCD08060466 | [Molecular Weight]
409.41 | [MOL File]
70458-96-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Off-white to light yellow cryst powder | [Melting point ]
220°C | [Boiling point ]
555.8±50.0 °C(Predicted) | [density ]
1.2504 (estimate) | [storage temp. ]
2-8°C
| [solubility ]
Very slightly soluble in water, slightly soluble in acetone and in ethanol (96 per cent). | [form ]
Crystalline Powder | [pka]
pKa1 6.34; pKa2 8.75(at 25℃) | [color ]
White to yellow | [Water Solubility ]
Soluble in acetic acid. Also soluble in acetone or cloroform. Slightly soluble in water | [Usage]
An antibacterial. Fluorinated quinolone antibacterial | [Merck ]
14,6700 | [Stability:]
Hygroscopic | [InChI]
InChI=1S/C16H18FN3O3/c1-2-19-9-11(16(22)23)15(21)10-7-12(17)14(8-13(10)19)20-5-3-18-4-6-20/h7-9,18H,2-6H2,1H3,(H,22,23) | [InChIKey]
OGJPXUAPXNRGGI-UHFFFAOYSA-N | [SMILES]
N1(CC)C2=C(C=C(F)C(N3CCNCC3)=C2)C(=O)C(C(O)=O)=C1 | [CAS DataBase Reference]
70458-96-7(CAS DataBase Reference) | [EPA Substance Registry System]
3-Quinolinecarboxylic acid, 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)- (70458-96-7) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S37/39:Wear suitable gloves and eye/face protection . | [WGK Germany ]
2
| [RTECS ]
VB2005000
| [HazardClass ]
IRRITANT | [HS Code ]
29335900 | [Hazardous Substances Data]
70458-96-7(Hazardous Substances Data) | [Toxicity]
LD50 in mice, rats (mg/kg): >4000 orally (both species); 1500 s.c. (both species); 470, >500 i.m.; 220, 270 i.v. (Irikura) |
| Hazard Information | Back Directory | [Description]
Norfloxacin is the first of the third generation nalidixic acid analogs to reach the
marketplace. It exhibits potent in vitro and in vivo activity against Pseudomonas,
enteric gram-negative rods and gram-positive cocci. Norfloxacin is orally
effective in the treatment of urinary tract infections, including those due to
organisms refractory to many other agents. | [Originator]
Kyorin (Japan) | [Definition]
ChEBI: A quinolinemonocarboxylic acid with broad-spectrum antibacterial activity against most gram-negative and gram-positive bacteria. Norfloxacin is bactericidal and its mode of action depends on blocking of bacterial DNA replication by binding itself to an enz
me called DNA gyrase. | [Manufacturing Process]
36 g (0.134 mol) of 7-chloro-1-ethyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3carboxylic acid, 46 g of piperazine and 210 cm3 of pyridine were heated under reflux for 6 hours, while stirring. After the starting material had dissolved, a precipitate appeared after heating for about 2 hours 30 minutes. The major part of the solvent was removed by concentration in vacuo (15 mm Hg; 100°C). In order to remove the pyridine as completely as possible, the residue was taken up in 200 cm3of water and the concentration in vacuo was repeated.
The residue, resuspended in 150 cm3 of water, was stirred. 150 cm3 of 2N NaOH were added thereto. The solution, which was slightly turbid, was treated with 5 g of animal charcoal and stirred for 30 minutes. After filtration, the pH was brought to 7.2 by adding acetic acid while stirring. The precipitate was filtered off, washed with water and dissolved in 250 cm3 of a 10% aqueous acetic acid. The acid solution (pH 4.4) was filtered and then brought to pH 7.2 by gradually added 2N NaOH.
The suspension was heated to 90°C, while stirring. The crystals were separated and recrystallized from 280 cm3 of a mixture of DMF (1 volume) and ethanol (4 volumes). After drying in vacuo over phosphorus pentoxide, 29.5 g (yield 70%) of 1-ethyl-6-fluoro-4-oxo-7-piperazinyl-1,4dihydroquinoline-3-carboxylic acid, melting point 222°C, were obtained.
In air, this product is hygroscopic and gives a hemihydrate.
| [Brand name]
Chibroxin (Merck); Noroxin (Merck). | [Therapeutic Function]
Antibacterial | [Antimicrobial activity]
It is active against a wide range of Gram-negative bacteria, including Enterobacteriaceae and Campylobacter spp. Ps. aeruginosa, Acinetobacter, Serratia and Providencia spp. are weakly susceptible (and often resistant). It has no useful activity against anaerobes, Chlamydia, Mycoplasma and Mycobacterium spp. | [Pharmaceutical Applications]
A 6-fluoro, 7-piperazinyl quinoline available for oral administration and as an ophthalmic ointment. | [Pharmacokinetics]
Oral absorption: 50–70%
Cmax 400 mg oral :1.5 mg/L after 1–1.5 h
Plasma half-life :3–4 h
Volume of distribution: 2.5–3.1 L/kg
Plasma protein binding: 15%
absorption and distribution
Norfloxacin displays linear kinetics. There is no significant accumulation with the recommended dosage of 400 mg every 12 h. Food slightly delays but does not otherwise impair absorption. Antacids reduce absorption. It is widely distributed, but concentrations in tissues other than those of the urinary tract are low: levels in the prostate are around 2.5 mg/g.
Metabolism and excretion
Six or more inactive metabolites are produced. Around 30% of a dose appears as unchanged drug in the urine and <10% as metabolites, producing peak concentrations of microbiologically active drug of around 100–400 mg/L. Urinary recovery is halved by probenecid, with little effect on the plasma concentration. The apparent plasma elimination half-life increases with renal impairment, rising to around 8 h in the anuric patient. Some of the drug appears in the bile where concentrations three- to seven-fold greater than the simultaneous plasma levels are achieved, but this is not a significant route of elimination and hepatic impairment is without effect. Very variable quantities, averaging 30% of a dose, appear in the feces, producing concentrations of active agent of around 200–2000 mg/kg. | [Clinical Use]
Complicated and uncomplicated urinary tract infections (including prophylaxis in recurrent infections), prostatitis
Uncomplicated gonorrhea
Gastroenteritis caused by Salmonella, Shigella and Campylobacter spp., Vibrio cholerae
Conjunctivitis (ophthalmic preparation) | [Side effects]
Untoward reactions are those common to the fluoroquinolones. Gastrointestinal tract disturbances, which are generally mild, have been reported in 2–4% of patients. CNS disturbances have largely been limited to headache, drowsiness and dizziness. Co-administration with theophylline results in increased plasma theophylline levels. | [Safety Profile]
Poison by intravenous route.Moderately toxic by other routes. Human systemic effectsby ingestion: musculoskeletal changes. An experimentalteratogen. Other experimental reproductive effects.Mutation data reported. When heated to decomposition itemits | [Synthesis]
Norfloxacin, 1-ethyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-
quinolincarboxylic acid (33.2.18), is the first representative of a series of fluorinated
quinolones as well as the first drug of the quinolone derivatives used in medicine that con�tains a piperazine substituent. The method of synthesis is basically the same as that sug�gested for synthesizing nalidixic and oxolinic acids.
Reacting 3-chloro-4-fluoroaniline and ethyl ethoxymethylenmalonate gives the substi�tution product (33.2.15), which upon heating in diphenyl ester cyclizes into ethyl ester of
6-fluoro-7-chloro-1,4-dihydro-3-quinolin-4-on-carboxylic acid (33.2.16). Direct treatment
of the product with ethyl iodide in the presence of triethylamine and subsequent hydro�lysis with a base gives 1-ethyl-6-fluoro-7-chloro-1,4-dihydro-3-quinolin-4-on-carboxylic
acid (33.2.17). Reacting this with piperazine gives norfloxacin (33.2.18).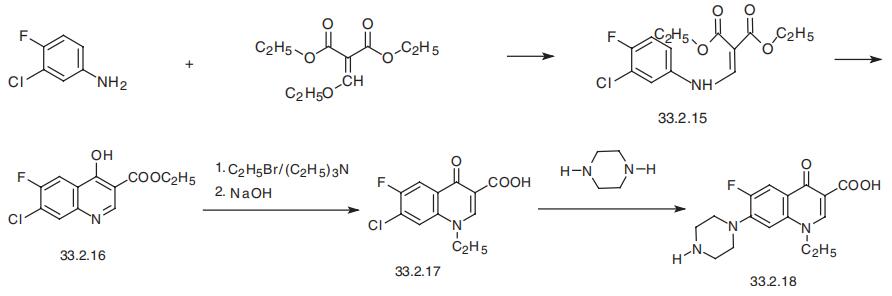
| [Drug interactions]
Potentially hazardous interactions with other drugs
Aminophylline: possibly increased risk of
convulsions, increased levels of aminophylline.
Analgesics: increased risk of convulsions with
NSAIDs.
Anticoagulants: anticoagulant effect of coumarins
enhanced.
Antimalarials: manufacturer of artemether with
lumefantrine advises avoid.
Ciclosporin: increased risk of nephrotoxicity.
Muscle relaxants: possibly increases tizanidine
concentration.
Theophylline: possibly increased risk of convulsions;
increased levels of theophylline. | [Metabolism]
Some metabolism occurs, possibly in the liver.
Norfloxacin is eliminated through metabolism, biliary
excretion and renal excretion. Renal excretion occurs by
both glomerular filtration and net tubular secretion. In
the first 24 hours, 33-48% of the drug is recovered in the
urine.
Norfloxacin exists in the urine as norfloxacin and six
active metabolites of lesser antimicrobial potency.
The parent compound accounts for over 70% of total
excretion. About 30% of an oral dose appears in the
faeces. | [storage]
Store at -20°C | [References]
[1] R W DARRELL C L F S M Modak. Norfloxacin and silver norfloxacin in the treatment of Pseudomonas corneal ulcer in the rabbit.[J]. Transactions of the American Ophthalmological Society, 1984, 82: 75-91.
[2] ELIAS GEBRU AWJI. The in vitro activity of 15 antimicrobial agents against bacterial isolates from dogs.[J]. Journal of Veterinary Medical Science, 2012, 74 8: 1091-1094. DOI: 10.1292/jvms.12-0043
[3] F SORIANO. Treatment of encrusted cystitis caused by Corynebacterium group D2 with norfloxacin, ciprofloxacin, and teicoplanin in an experimental model in rats.[J]. Antimicrobial Agents and Chemotherapy, 1991, 35 12: 2587-2590. DOI: 10.1128/aac.35.12.2587 |
| Questions And Answer | Back Directory | [Anti-infection drug]
Norfloxacin is a quinolone-class anti-infective drug with high degree of antibacterial activity against Escherichia coli, Shigella, Salmonella, Proteus, Pseudomonas aeruginosa and other gram-negative bacteria as well as excellent antibacterial effect against Staphylococcus aureus, pneumococcus bacteria and other Gram-positive bacteria. Its major site of action is in the bacterial DNA gyrase, causing the rapid cracking of the bacteria DNA helix and rapidly inhibiting the bacterial growth and reproduction, finally killing the bacteria. Moreover, it has a strong penetration capability into the cell walls so that it has a stronger bactericidal effect with a small stimulation on the gastric mucosa.
It is clinically used for treating the susceptible strains caused infection diseases in urinary tract, intestinal, ENT, gynecology, surgery and dermatology with the major indications as follows:
1, genitourinary infections: simple and complicated urinary tract infections, bacterial prostatitis, gonococcal urinary tract infections and reproductive tract infections.
2, gastrointestinal tract infections.
3, typhoid and other Salmonella infections.
Norfloxacin is a concentration-dependent drug which means that the in vivo concentration of the drug is directly proportional to the in vivo efficacy of the drug. The higher the concentration is, the better effect is. As the food will slow down the absorption rate of norfloxacin in the human body, the plasma concentration in the case of taking it before meals (with empty stomach) is 2-3 fold of that in the case of taking it after a meal; therefore, taking it after the meal will greatly reduce the efficacy and may also lead to the drug resistance issue of the susceptible bacteria to the norfloxacin and other similar antimicrobial drugs.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
| [Chemical Properties]
It is white to light yellow crystalline powder. It is odorless with slightly bitter taste. Upon exposure to the air, it can easily absorb moisture to form a hemihydrate. Its color will be deepened upon light. Solubility (mg/m1) in 25 ℃: 0.28 in water, 0.98 in methanol, 1.9 in ethanol, 5.1 in acetone, 5.5 in chloroform, 0.01 in diethyl ether, 0.15 in benzene, 0.94 in ethyl acetate, 5.1 in octanol, and 340 in glacial acetic acid. It is easily soluble in acidic or alkaline solution and slightly soluble in dimethylformamide. Its solubility in the water depends on the pH value and increases rapidly within the range of at pH <5 or pH> 10. It has a melting point of 218-224 ℃. It has also been reported of a melting point of 220-221 ℃. The maximum UV absorption (0.1mol/L sodium hydroxide solution): about 274,325.336nm (A has a height of about 1109, 437, 425). pKal 6.34; pKa2 8.75. Acute toxicity LD50 in mice, rats (mg/kg): All> 4000 oral administration; all hypodermic 1500; 470,> 500 intramuscular injection; 220,270 intravenous injection.
| [Uses]
Norfloxacin belongs to third-generation quinolone antibacterial agent developed by Japanese Kyorin Company in 1978. It has features of broad antibacterial spectrum and strong antibacterial activity. It has a strong antibacterial effect against Escherichia coli, pneumobacillus, Aerobacter aerogenes, and Aerobacter cloacae, Proteus, Salmonella, Shigella, Citrobacter and Serratia. It is clinically used for treating the susceptible strain’s causing infections of urinary system, intestinal, respiratory system, surgery, gynecology, ENT and dermatology. It can also be used for the treatment of gonorrhea.
| [Production method]
Nitration of o-dichlorobenzene or the chlorination of nitro chlorobenzene can both generate 3, 4-dichloro-nitrobenzene. It then undergoes reflux with potassium fluoride in dimethyl sulfoxide for being fluorinated to give 3-chloro-4-fluoro-nitrobenzene. In the presence of hydrochloric acid or aqueous acetic acid, it is further reduced by iron to 3-chloro-4-fluoro-aniline. 3-chloro-4-fluoro-aniline was then subject to reflux together with triethyl orthoformate and diethyl malonate (generate diethyl ethoxymethylenemalonate) in the presence of ammonium nitrate to give the condensation product with heating and cyclization in diphenyl ether or liquid paraffin to form the 7-chloro-6-fluoro-4-hydroxyquinoline-3-carboxylate with ethylation and further hydrolyzation to obtain the ethylated product. Finally, the ethylated product is condensed with piperazine to obtain norfloxacin. Its technology is relatively mature with a relative high yield being generally 40% to 65%. However, when introducing the piperazinyl group to the 7 position, the byproduct with the fluorine atom in 6 position can account for about 25%. It is hard for separation that can affect the yield. The overall yield calculated based on nitro chlorobenzene is above 8%.
Before the introduction of the pyrazine ring, 1-ethyl-6-fluoro-7-chloro-1,4-dihydro-4-oxo-quinoline-8-carboxylic acid ethyl ester should first react with fluoroboric acid or a boron trifluoride-diethyl ether or boron acetate to have the carbonyl group in 4 position form boron chelate. Further re-introduction of pyrazinyl can reduce the side reactions of the displacement of the position 7’s fluorine and can increase the yield by 15%, as well as improve the quality of the product.
There are many studies regarding the synthesis of norfloxacin at home and abroad. But there have not been too many way for being used in industrial production. The improvement of its synthesis route can be mainly reflected in two aspects. First, improve the process of forming a ring; the second is doing sth on the introduction of piperazine group.
| [Category]
toxic substances
| [Toxicity grading]
poisoning
| [Acute toxicity]
intravenous-rat LD50: 245 mg/kg; Oral-Mouse LD50: 4000 mg/kg
| [Flammability and hazardous characteristics]
it is combustible with combustion produces toxic fumes of nitrogen oxides and fluorides; Side effect when patients take it: musculoskeletal functional changes
| [Storage Characteristics]
ventilation low-temperature and dry
| [Extinguishing agent]
Dry powder, foam, sand, carbon dioxide, water spray
|
|
|