| Identification | More | [Name]
Azelnidipine | [CAS]
123524-52-7 | [Synonyms]
2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-[1-(diphenylmethyl)-3-azetidinyl] 5-(methylethyl) ester
AZELNIDIPINE
3-[1-(Diphenylmethyl)-3-azetidinyl]-5-(1-methylethyl-2-amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylate
AzelnidipineC33H34N406
AZELINIDIPINE
AZELNIDIPINE(FORR&DONLY)
2-Amino-1,4-dihydro-6-methyl-4-(3-nitrophenyl)-3,5-pyridinedicarboxylic acid 3-[1-(diphenylmethyl)-3-azetidinyl] 5-(methylethyl) ester
CS-905, RS-9054, 2-Amino-1,4-dihydro-6-methyl-4-(3 nitrophenyl)-3,5-pyridinedicarboxylic acid 3-[1-(diphenylmethyl)-3-azetidinyl] 5-(1-methylethyl) ester
Azelidipine
Azenildipine
Calblock
CS-905 | [EINECS(EC#)]
634-143-0 | [Molecular Formula]
C33H34N4O6 | [MDL Number]
MFCD00865803 | [Molecular Weight]
582.65 | [MOL File]
123524-52-7.mol |
| Chemical Properties | Back Directory | [Melting point ]
120-126°C | [Boiling point ]
709.3±60.0 °C(Predicted) | [density ]
1.33±0.1 g/cm3(Predicted) | [storage temp. ]
Room temp | [solubility ]
DMSO: >10mg/mL | [form ]
powder | [pka]
7.89(at 25℃) | [color ]
Yellow | [Merck ]
14,907 | [InChIKey]
ZKFQEACEUNWPMT-UHFFFAOYSA-N | [SMILES]
C1(N)NC(C)=C(C(OC(C)C)=O)C(C2=CC=CC([N+]([O-])=O)=C2)C=1C(OC1CN(C(C2=CC=CC=C2)C2=CC=CC=C2)C1)=O | [CAS DataBase Reference]
123524-52-7(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
Xn | [Risk Statements ]
R22:Harmful if swallowed.
R41:Risk of serious damage to eyes. | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S39:Wear eye/face protection . | [WGK Germany ]
3 | [RTECS ]
US7968332 | [HS Code ]
2933.79.1500 | [Toxicity]
LD50 in female, male mice, female, male rats (mg/kg): 785, 979, 1267, 1971 orally |
| Hazard Information | Back Directory | [Description]
Azelnidipine, a member of the 1,4-dihydropyridine class of L-type calcium channel
blockers with a slow onset profile, was marketed in Japan for the treatment of
hypertension. Azelnidipine is synthesized via the condensation of iso-propyl 2-(3-
nitrobenzylidene)acetoacetate with (1-diphenylmethylazetidin-3-yl)-3,3-diamino acrylate.
The diamino acrylate intermediate is prepared from the cyanoacetic ester by sequential treatment with HCl and ammonia. In receptor binding studies using
porcine heart membrane fractions, azelnidipine exhibits an IC50 of 3.1 nM and an
apparent Ki of 2.1 nM. Its tight binding and slow onset are correlated with its high
lipophilicity. A slow onset is also noted in vitro in a rat aortic strip contraction assay,
and this effect continues after removal of the drug from the bath solution. In the
conscious spontaneously hypertensive rat (SHR) model of hypertension, it was more
potent that nicardipine and also had a more gradual onset and long-lasting effect. This
effect was noted both when dosed orally or intravenously. When SHR dosed at 1 or
3 mpk/day for 15 weeks, a sustained reduction in systolic blood pressure was noted (19
and 43 mmHg reduction, respectively). Cardiac output was increased and total
peripheral resistance was decreased in each group. Clinical studies of patients with
mild-to-moderate hypertension have shown that long-term treatment with azelnidipine
provided a sustained decrease in blood pressure (mean reduction systolic /diastolic:
27.8/16.6 mmHg). It similarly controlled blood pressure, as did amlodipine at 24 h. It
possesses a gradual onset of activity with plasma levels increasing before the
antihypotensive effect is attained. After plasma levels drop, the pharmacodynamic effect
is sustained. In clinical studies, azelnidipine did not show reflex tachycardia, a common
side effect of this class. Most common side effects were facial flushing and headache,
similar to other dihydropyridines. Azelnidipine is dosed orally once daily (8–16 mg), is
rapidly absorbed in a dose-dependent fashion, and has a mean terminal half-life of
19.2 h (8 mg dosage p.o. for seven days). Uniquely, it possesses a 2-amino function
associated with a longer half-life than related agents wherein this moiety is a methyl.
The very highly lipophilic 3-carboxylic ester side-chain is purported to contribute to the
gradual onset of activity and prolonged pharmacodynamic effect, unlike other drugs in
this class. This compound exhibits a much less pronounced first-pass metabolic effect
than nicardipine. | [Chemical Properties]
Yellow Solid | [Originator]
Sankyo (Japan) | [Uses]
Azelnidipine is a dihydropyridine calcium channel blocker with antihypertensice activity. Azelnidipine is used for treating ischemic heart disease and cardiac remodeling after myocardial infarction. S
tudies show that Azelnidipine ttreatment can reduce the risk of hyperglycemia induced metabolic disorders | [Application]
Azelnidipine is a novel dihydropyridine derivative, a L-type calcium channel blocker, and an antihypertensive. Acute administration of azelnidipine prevents a sudden drop of cardiac function after acute stress. Azelnidipine may have a protective role in inflammation associated with atherosclerosis. | [Definition]
ChEBI: Azelnidipine is an isopropyl ester. | [Brand name]
Calblock | [Biological Activity]
Azelnidipine, a novel dihydropyridine derivative, is a L-type calcium channel blocker and antihypertensive. Unlike other L-type calcium channel blockers, azelnidipine causes minimal stimulation of the sympathetic nervous system despite its significant depressor effect. Azelnidipine may have a protective role in inflammation in atherosclerosis. | [Synthesis]
A solution of benzhydrylamine
(46) and epichlorohydrin (47) was mixed without adding
solvent to give azetidinol 48 in 57% yield. DCC
coupling between cyanoacetic acid (49) and azetidinol 48 in
hot THF gave ester 50 in 93% yield. Cyanoester 50 was
treated with ethanol and HCl gas in chloroform to give
imidate HCl salt 51, which was treated with ammonia gas in
chloroform and ammonium acetate in acetonitrile to give the
corresponding amidinoacetate 52. A modified Hantzsch
reaction was employed to construct the 2-amino-1,4-
dihydropyridine core structure. Compound 52 was
condensed with 2-(3-nitrobenzylidene)acetic acid isopropyl
ester (55) in the presence of NaOMe in refluxing isopropanol
to give the cyclized product, azelnidipine (V) in 74% yield.
Benzylideneacetoacetate 55 was obtained through the
Knoevenagel reaction employing 3-nitrobenzaldehyde (53)
and isopropyl acetoacetate (54) in isopropanol containing a
catalytic amount of piperidinium acetate at 45-55oC in 65%
yield.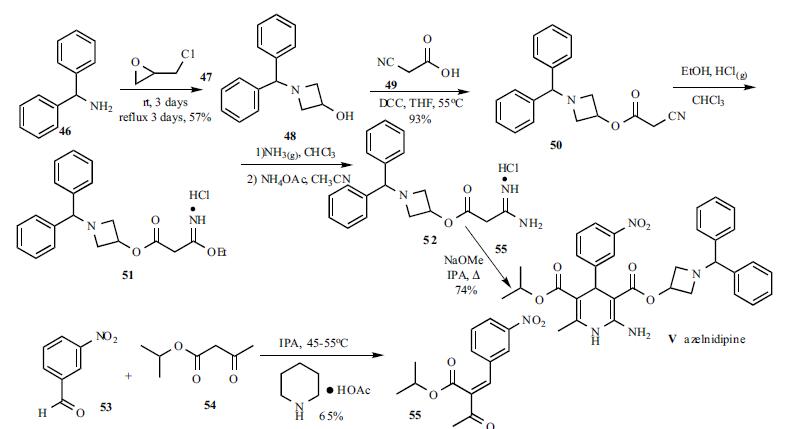
| [storage]
Store at -20°C |
|
|