| Identification | More | [Name]
Fenoterol | [CAS]
13392-18-2 | [Synonyms]
1-(3,5-dihydroxyphenyl)-1-hydroxy-2-[(4-hydroxyphenyl)isopropylamino]ethane
5-[1-hydroxy-2-[[2-(4-hydroxyphenyl)-1-methylethyl]amino]ethyl]-1,3-benzenediol
AURORA KA-7877
FENOTEROL
1-(p-hydroxyphenyl)-2-((beta-hydroxy-beta-(3’,5’-dihydroxyphenyl))ethyl)amin
1,3-benzenediol,5-(1-hydroxy-2-((2-(4-hydroxyphenyl)-1-methylethyl)amino)eth
3,5-dihydroxy-alpha-(((p-hydroxy-alpha-methylphenethyl)amino)methyl)benzyla
benzylalcohol,3,5-dihydroxy-alpha-(((p-hydroxy-alpha-methylphenethyl)amino)me
lcohol
opropane
1-(3,5-Dihydroxyphenyl)-1-hydroxy-2-[1-(4-hydroxyphenyl)isopropyl]aminoethane
1-(3,5-Dihydroxyphenyl)-2-(p-hydroxy-α-methylphenethylamino)ethanol
1-(3,5-Dihydroxyphenyl)-2-[[1-(4-hydroxybenzyl)ethyl]amino]ethanol
1,3-Benzenediol, 5-[1-hydroxy-2-[[2-(4-hydroxyphenyl)-1-methylethyl]amino]ethyl]-
3,5-Dihydroxy-α-[p-hydroxy-α-methylphenethylamino]methylbenzyl alcohol
Benzyl alcohol, 3,5-dihydroxy-α-[[(p-hydroxy-α-methylphenethyl)amino]methyl]-(8CI)
Phenoterol
Th 1165
3,5-Dihydroxy-α-[[(4-hydroxy-α-methylphenethyl)amino]methyl]benzyl alcohol | [EINECS(EC#)]
680-817-2 | [Molecular Formula]
C17H21NO4 | [MDL Number]
MFCD00242675 | [Molecular Weight]
303.35 | [MOL File]
13392-18-2.mol |
| Hazard Information | Back Directory | [Originator]
Berotec,Boehringer Ingelheim,W. Germany,1972 | [Uses]
antiinflammatory | [Definition]
ChEBI: A member of the class resorcinols that is 5-(1-hydroxyethyl)benzene-1,3-diol in which one of the methyl hydrogens is replaced by a 1-(4-hydroxyphenyl)propan-2-amino group. A beta2-adrenergic agonist, it is used (as the
ydrobromide salt) as a bronchodilator in the management of reversible airway obstruction. | [Manufacturing Process]
441 grams (1.4 mols) of 3,5-diacetoxy-α-bromo-acetophenone (MP 66°C),
prepared by bromination of 3,5-diacetoxy-acetophenone, were added to a
solution of 714 grams (2.8 mols) of 1-p-methoxyphenyl-2-benzylaminopropane
in 1,000 cc of benzene, and the resulting solution mixture was
refluxed for 1 hour. The molar excess of 1-p-methoxy-phenyl-2-benzylaminopropane
precipitated out as its hydrobromide. After separation of the
precipitated hydrobromide of the amino component, the hydrochloride of 1-pmethoxy-
phenyl-2-(β-3',5'-diacetoxyphenyl-β-oxo)-ethyl-benzylamino-propane
was precipitated from the reaction solution by addition of an ethanolic solution
of hydrochloric acid. The precipitate was separated and, without further
purification, was deacetylated by boiling it in a mixture of 2 liters of aqueous
10% hydrochloric acid and 1.5 liters of methanol.
The resulting solution was filtered through animal charcoal and, after addition
of 2 liters of methanol, it was debenzylated by hydrogenation at 60°C over
palladinized charcoal as a catalyst. After removal of the catalyst by filtration,
the filtrate was concentrated by evaporation, whereupon the hydrochloride of
1-p-methoxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-ethylamino-propane
(MP 244°C) crystallized out. For the purpose of demethylation, the 350 grams
of the hydrochloride thus produced were refluxed for 2 hours with 3.5 liters of
aqueous 48% hydrobromic acid. Upon cooling of the reaction solution, 320
grams of 1-p-hydroxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-ethylaminopropanehydrobromide
(MP 220°C) crystallized out.
·220 grams of 1-p-hydroxyphenyl-2-(β-3',5'-dihydroxyphenyl-β-oxo)-
ethylamino-propane hydrobromide were dissolved in 1 liter of methanol, the
resulting solution was boiled with activated charcoal, the charcoal was filtered
off and the filtrate was hydrogenated in the presence of Raney nickel at 60°C
and 5 atmospheres gauge. Thereafter, the catalyst was filtered off, the
methanolic solution was admixed with a small amount of concentrated
hydrobromic acid, and the mixture was evaporated to dryness in vacuo. The
residue was stirred with acetone, the mixture was vacuum filtered and the
filter cake was recrystallized from a mixture of methanol and ether. The 1-phydroxyphenyl-
2-(β-3',5'-dihydroxyphenyl-β-hydroxy)-ethylamino-propane
hydrobromide thus obtained had a melting point of 222° to 223°C. | [Brand name]
Berotec [as hydrobromide](Boehringer Ingelheim);Dosberotec;Duovent;Fensol;Partusisten. | [Therapeutic Function]
Bronchodilator | [World Health Organization (WHO)]
Fenoterol, a beta 2-adrenoreceptor agonist with bronchodilator
activity, was introduced in 1971 for the management of asthma. In the 1960's, the
use of other sympathomimetics in pressurised aerosols had already been
associated with an increase in mortality due to asthma. However, it was not clear
whether patients died from the severity of the asthma attack or from its treatment. | [Mechanism of action]
Fenoterol is a selective stimulant of β2-adrenoreceptors. It dilates bronchi and blood vessels,
has a pronounced tocolytic action, lowers contractile activity and reduces uterus
tonicity. It is mainly used in premature births. | [Clinical Use]
Fenoterol is a selective β2-sympathomimetic
agent that is in wide clinical use in Europe. | [Synthesis]
Fenoterol, 3,5-dihydroxy-|á[[(-p-hydroxy-|á-methylphenethyl)amino]methyl]-
benzyl alcohol (23.3.16), is synthesized from 3,5-diacetoxyacetophenone, which is brominated
to give 3,5-diacetoxybromacetophenone (23.3.12). This is reacted with
2-benzylamino-1-(4-methoxyphenyl)-propane, giving the corresponding tertiary amine
23.3.13. Hydrolysis of the acetyl group of this product and removal of the protective benzyl
group by hydrogen reduction using a palladium on carbon catalyst gives a secondary
amine 23.3.14. This is reacted with hydrobromic acid, which cleaves the ether bond in the
benzene ring, producing phenol derivative 23.3.15. Finally, reduction of the carbonyl group
with hydrogen gives the desired fenoterol (23.3.16).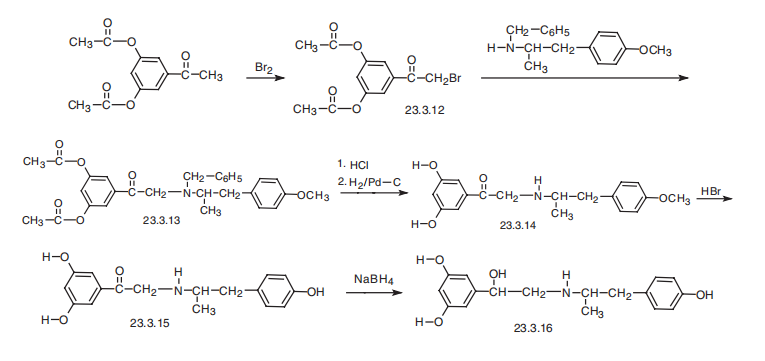
|
|
| Company Name: |
LGM Pharma
|
| Tel: |
1-(800)-881-8210 |
| Website: |
www.lgmpharma.com |
|