| Identification | More | [Name]
Bis(triphenylphosphine)nickel(II)chloride | [CAS]
14264-16-5 | [Synonyms]
BIS(TRIPHENYLPHOSPHINE)NICKEL(2) DICHLORIDE
BIS(TRIPHENYLPHOSPHINE)NICKEL(II) CHLORIDE
BIS(TRIPHENYLPHOSPHINE)NICKEL(II) DICHLORIDE
BIS(TRIPHENYLPHOSPHINO)NICKEL(II) CHLORIDE
DICHLOROBIS(TRIPHENYLPHOSPHINE)NICKEL
DICHLOROBIS(TRIPHENYLPHOSPHINE)NICKEL(II)
NICKEL BIS(TRIPHENYLPHOSPHINE)DICHLORIDE
NICKEL(II)-BIS(TRIPHENYLPHOSPHINE) DICHLORIDE
TRIPHENYLPHOSPHINE NICKEL (II) CHLORIDE
Bis(tri-N-butylphosphine)dichloronickel
bis(triphenylphosphine)dichloro-nicke
Bis(triphenylphosphine)dichloronickel
Bis(triphenylphosphine)nickel dichloride
Dichlorobis(triphenyIphosphine)-nickel(II)
dichlorobis(triphenylphosphine)-nicke
Nickel, bis(triphenylphosphine)dichloro-
Nickel, dichlorobis(triphenylphosphine)-
Phosphine, tributyl-, compd. with nickelchloride (2:1)
tributyl-phosphincompd.withnickelchloride(2:1)
Bis(triphenylphosphine)nickel(II) chloride, 98+% | [EINECS(EC#)]
238-154-8 | [Molecular Formula]
C36H30Cl2NiP2 | [MDL Number]
MFCD00009592 | [Molecular Weight]
654.17 | [MOL File]
14264-16-5.mol |
| Chemical Properties | Back Directory | [Appearance]
dark green to dark grey crystals or powder | [Melting point ]
250 °C (dec.)(lit.) | [storage temp. ]
Inert atmosphere,Room Temperature | [solubility ]
DMSO (Slightly), Methanol (Slightly) | [form ]
Crystals or Powder | [color ]
Dark green to dark gray | [Water Solubility ]
insoluble | [Sensitive ]
Hygroscopic | [Exposure limits]
NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 | [InChI]
InChI=1S/2C18H15P.2ClH.Ni/c2*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h2*1-15H;2*1H;/q;;;;+2/p-2 | [InChIKey]
ZBRJXVVKPBZPAN-UHFFFAOYSA-L | [SMILES]
P(C1C=CC=CC=1)(C1=CC=CC=C1)C1C=CC=CC=1.P(C1C=CC=CC=1)(C1C=CC=CC=1)C1C=CC=CC=1.[Ni](Cl)Cl | [CAS DataBase Reference]
14264-16-5(CAS DataBase Reference) | [NIST Chemistry Reference]
dichlorobis(triphenylphosphine)nickel(14264-16-5) | [Storage Precautions]
Store under nitrogen | [EPA Substance Registry System]
Nickel, dichlorobis(triphenylphosphine)- (14264-16-5) |
| Safety Data | Back Directory | [Hazard Codes ]
T | [Risk Statements ]
R45:May cause cancer.
R43:May cause sensitization by skin contact.
R52/53:Harmful to aquatic organisms, may cause long-term adverse effects in the aquatic environment .
R34:Causes burns.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S53:Avoid exposure-obtain special instruction before use .
S36/37:Wear suitable protective clothing and gloves .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) .
S60:This material and/or its container must be disposed of as hazardous waste .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice . | [RIDADR ]
3260 | [WGK Germany ]
3
| [RTECS ]
QR6170000
| [F ]
21 | [TSCA ]
Yes | [HazardClass ]
6.1(b) | [PackingGroup ]
III | [HS Code ]
29310095 | [Storage Class]
6.1D - Non-combustible acute toxic Cat.3
toxic hazardous materials or hazardous materials causing chronic effects | [Hazard Classifications]
Aquatic Chronic 3
Carc. 1B
Skin Sens. 1 |
| Questions And Answer | Back Directory | [Reaction]
- Catalyst for hydrosilylation of styrene with diphenysilane
- Catalyst for carboxylation of various aryl chlorides and other derivatives
- Catalyst for C–P cross-coupling reactions of diphenylphosphine oxide with aryl chloride
- Catalyst for N?Heterocyclic carbene-assisted cross-coupling reactions of diarylborinic acids with aryl chlorides,tosylates, and sulfamates.
- Catalyst for Negishi biaryl ketone synthesis by cross-coupling of amides with aryl zinc halides via carbon-nitrogen bond cleavage.
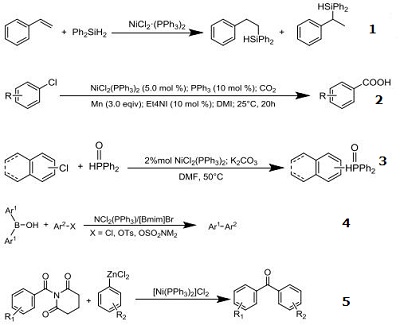
|
| Hazard Information | Back Directory | [Chemical Properties]
dark green to dark grey crystals or powder | [Uses]
Coordination compund. | [Uses]
Dichlorobis(triphenylphosphine)nickel(II) is used as a catalyst for cross-coupling of Grignard reagents, hydrosilylations, hydrogenation and polymerization. | [Uses]
suzuki reaction | [reaction suitability]
core: nickel
reagent type: catalyst | [Purification Methods]
Wash it with glacial AcOH and dry it in a vacuum over H2SO4 and KOH until AcOH is removed. [Venanzi J Chem Soc 719 1958, Kocienski et al. J Org Chem 54 1215 1989, Beilstein 16 IV 953.] |
|
|