| Identification | More | [Name]
Pemetrexed disodium | [CAS]
150399-23-8 | [Synonyms]
n-[4-[2-(2-amino-4,7-dihydro-4-oxo-1h-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-l-glutamic acid disodium salt
PEMETREXED
PEMETREXED DISODIUM
PEMETREXED DISODIUM(ALIMTA)
PEMETREXED SODIUM
premetred disodium
2-[4-[2-(4-Amino-2-oxo-3,5,7-triazabicyclo[4.3.0]nona-3,8,10-trien-9-yl)ethyl]benzoyl]aminopentanedioic acid disodium salt
PEMETREXED DISODIUM 99%
PEMETREXED DISODIUM 2.5H2O
PEMETREXED DISODIUM FOR INJECTION
PEMETREXED DISODIUM FOR INJECTION BK-A-01
Pemetrexed Dinatrium
ALIMTA
Alimt
LY-231514
N-[4-[(2-Amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic Acid Disodium
N-[4-[2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid disodium salt
Pemwtrexed Disodium | [EINECS(EC#)]
604-733-2 | [Molecular Formula]
C20H19N5Na2O6 | [MDL Number]
MFCD07779402 | [Molecular Weight]
471.37 | [MOL File]
150399-23-8.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline Solid | [Melting point ]
254-258°C (dec.) | [storage temp. ]
Keep in dark place,Inert atmosphere,Store in freezer, under -20°C | [solubility ]
Methanol, Water | [form ]
Solid | [color ]
Off-White | [Usage]
Multitargeted antifolate; inhibits thymidylate synthase as well as other folate dependent enzymes. Antineoplastic | [Stability:]
Hygroscopic | [CAS DataBase Reference]
150399-23-8(CAS DataBase Reference) |
| Safety Data | Back Directory | [Hazard Codes ]
T;Xi,Xi,T | [Risk Statements ]
R36/38:Irritating to eyes and skin .
R60:May impair fertility.
R61:May cause harm to the unborn child.
R68:Possible risk of irreversible effects. | [Safety Statements ]
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S53:Avoid exposure-obtain special instruction before use . |
| Questions And Answer | Back Directory | [Description]
Pemetrexed disodium (marketed as ALIMTA®, “pemetrexed”) is a novel, multi-targeted antifolate. It suppresses tumor growth by impeding both DNA synthesis and folate metabolism. Pemetrexed disodium has demonstrated promising clinical activity in a wide variety of solid tumors, including non-small cell lung, breast, mesothelioma, colorectal, pancreatic, gastric, bladder, cervix, and head and neck.
Pemetrexed disodium is approved to be used alone or with other drugs to treat malignant pleural mesothelioma in patients who cannot be treated with surgery and non-small cell lung cancer (certain types) in patients whose disease is locally advanced or has metastasized (spread to other parts of the body). Pemetrexed disodium is also being studied in the treatment of other types of cancer.
| [References]
[1] https://www.cancer.gov/about-cancer/treatment/drugs/pemetrexeddisodium
[2] Axel-R. Hanauske, V. Chen P. Paoletti, C. Niyikiza (2001) Pemetrexed Disodium: A Novel Antifolate Clinically Active Against Multiple Solid Tumors, The Oncologist, 6, 363-373
|
| Hazard Information | Back Directory | [Chemical Properties]
Crystalline Solid | [Originator]
Eli Lilly (US) | [Uses]
Multitargeted antifolate; inhibits thymidylate synthase as well as other folate dependent enzymes. Antineoplastic | [Uses]
Pemetrexed is a novel antifolate and antimetabolite for TS, DHFR and GARFT with Ki of 1.3 nM, 7.2 nM and 65 nM, respectively | [Definition]
ChEBI: An organic sodium salt that is the disodium salt of N-{4-[2-(2-amino-4-oxo-4,7-dihydro-1H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl}-L-glutamic acid. Inhibits thymidylate synthase (TS), 4
1 dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT). | [Brand name]
Alimta (Lilly). | [General Description]
The drug is available in a 100-mg sterile vial for IV use. Thedrug appears to be effective against a range of tumors includingmesothelioma, NSCLC, colorectal cancer, bladdercancer, and lung cancer. The mechanism of action involvesinhibition of TS resulting in inhibition of thymidylate andDNA synthesis. This drug is a pyrrolopyrimidine analog offolate with antifolate activity. Resistance can occur by increasedexpression of TS, decreased binding affinity for TS,or decreased drug transport into cells. The drug is administeredonly via the IV route and distributes to all tissues.Cellular activation to the more potent polyglutamated formsoccurs, and the majority of the dose is excreted unchangedin the urine. The drug interaction and toxicity profiles aresimilar to that of methotrexate. | [Mechanism of action]
Like methotrexate, it is actively transported into tumor cells through reduced folate carriers and, in polyglutamated form, inhibits the synthesis of pyrimidine and purine-based nucletotides by disrupting folate�dependent metabolic processes . In addition to DHFR, this pyrrolopyrimidine-based inhibitor binds tightly to thymidylate synthase and GAR transformylase. | [Clinical Use]
Pemetrexed is a novel multitarget antifolate used by the IV route for the treatment of advanced or metastatic nonsmall cell lung cancer and in combination with cisplatin in malignant pleural mesothelioma. | [Side effects]
Patients on pemetrexed must take folate and vitamin B12 supplements to reduce the risk of bone marrow suppression (neutropenia, thrombocytopenia, and anemia) and GI side effects. Pretreatment with corticosteroids can reduce the risk of drug-induced skin rash. Pemetrexed has a half-life of 3.5 hours and is excreted primarily unchanged via the kidneys. Significant cross-resistance has been noted between pemetrexed and other pyrimidine and folate antagonists. | [Synthesis]
A number of papers
outlining the syntheses of pemetrexed and related analogs have appeared. A practical and scalable synthetic
route is depicted in Scheme 9. Palladium (0) coupling
of methyl 4-bromobenzoate (57) with 3-butyn-1-ol (58) gave
crystalline 59, which was then reduced over palladium on
carbon in DCM to give alcohol 60. Filtration of the catalyst
afforded a DCM solution of alcohol 60, which was utilized
directly in a TEMPO-catalyzed sodium hypochlorite
oxidation, providing known aldehyde 61 without isolation.
Addition of 5,5-dibromobarbituric acid (DBBA) and
catalytic amount of HBr in acetic acid to the DCM solution
of 61 effected the conversion to a-bromoaldehyde 62. After
aqueous work-up, the solution was concentrated and diluted
with acetonitrile to exchange solvents. Addition of
commercially available 2,4-diamino-6-hydroxypyrimidine
(63), aqueous sodium acetate and heating to 45??C resulted in
cyclic condensation and precipitation of pyrrolo[2,3-
d]pyrimidine 64 from the reaction mixture in 67% yield
based on 60. Saponification of 64 with aqueous sodium
hydroxide followed by acidification afforded the carboxylic
acid derivative 65, which was elaborated to 66 by
chlorodimethoxytriazine active ester coupling method.
Reaction of 65 with 2-chloro-4,6-dimethoxy-1,3,5-triazine
(CDMT) in the presence of N-methylmorpholine in DMF
solution followed by reaction of the resulting dimethoxy-s-triazinyl ester with diethyl L-glutamate afforded crude 66,
which was isolated via crystallization as pTSA salt 67.
Saponification of 67 with aqueous sodium hydroxide
followed by acidification with HCl gave pemetrexed as the
free acid, which was crystallized as disodium salt form.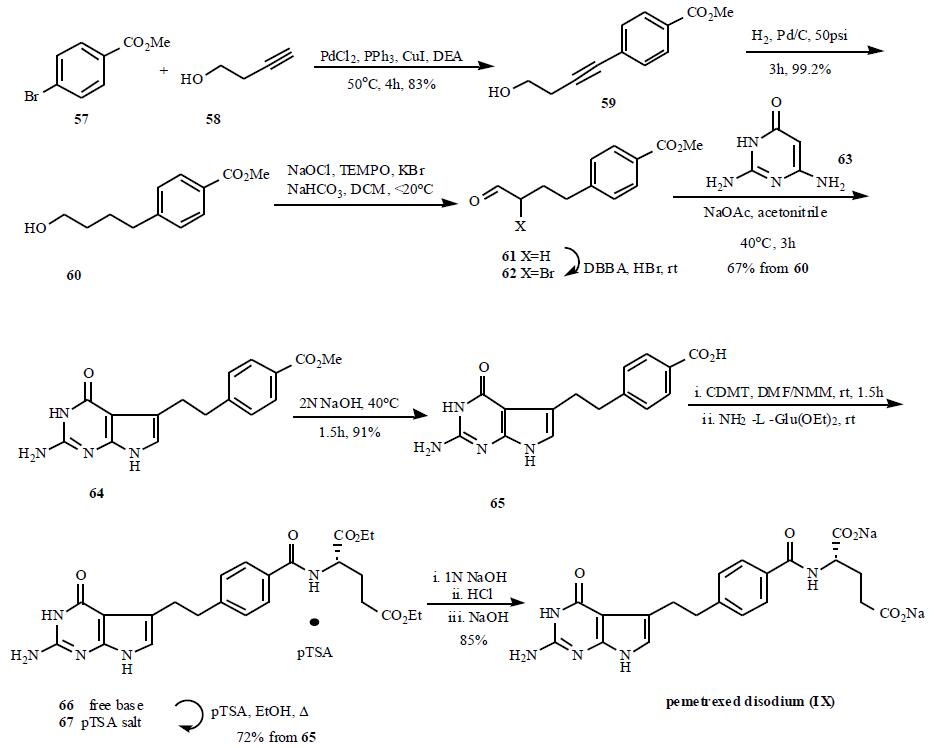
| [target]
MEK | ERK | [Drug interactions]
Potentially hazardous interactions with other drugs
Antimalarials: antifolate effect increased by
pyrimethamine.
Antipsychotics: avoid with clozapine, increased risk
of agranulocytosis.
Nephrotoxic agents: may reduce clearance of
pemetrexed - use with caution.
Live vaccines: avoid use; YELLOW
FEVER VACCINE ABSOLUTELY
CONTRAINDICATED. | [Metabolism]
Pemetrexed undergoes minimal hepatic metabolism,
and about 70-90% of a dose is eliminated unchanged in
the urine within 24 hours. In vitro studies indicate that
pemetrexed is actively secreted by OAT3 (organic anion
transporter). |
|
|