| Identification | More | [Name]
6-Thioguanine | [CAS]
154-42-7 | [Synonyms]
2-AMINO-6-MERCAPTOPURINE
2-AMINO-6-PURINETHIOL
2-aminopurine-6(1h)-thione
6-MERCAPTOGUANINE
6-THIOGUANINE
THIOGUANINE
2-amino-1,7-dihydro-6h-purin-6-thion
2-amino-1,7-dihydro-6h-purine-6-thion
2-amino-1,7-dihydro-6h-purine-6-thione
2-amino-6-merkaptopurin
2-amino-6-mp
2-aminopurin-6-thiol
2-amino-purine-6(1h)-thion
2-amino-purine-6-thio
2-aminopurine-6-thiol
6-mercapto-2-aminopurine
6-tg
bw5071
lanvis
nsc-752 | [EINECS(EC#)]
205-827-2 | [Molecular Formula]
C5H5N5S | [MDL Number]
MFCD00233553 | [Molecular Weight]
167.19 | [MOL File]
154-42-7.mol |
| Chemical Properties | Back Directory | [Appearance]
Crystalline, lyophilized, sterile, endot | [Melting point ]
≥300 °C(lit.) | [density ]
1.483 (estimate) | [refractive index ]
1.5605 (estimate) | [storage temp. ]
Store at RT. | [solubility ]
Practically insoluble in water and in ethanol (96%). It dissolves in dilute solutions of alkali hydroxides. | [Boiling point ]
555.4±42.0 °C(Predicted) | [form ]
lyophilized powder
| [pka]
pKa 8.22 (Uncertain) | [color ]
Yellow to green | [biological source]
synthetic (organic) | [Water Solubility ]
soluble | [Usage]
The compound produced dose-dependent inhibition of stimulated expression of TRAIL protein | [Detection Methods]
NMR,MS,UV | [Merck ]
14,9337 | [BRN ]
157765 | [Stability:]
Stable for 1 year from date of purchase as supplied. Solutions in DMF may be stored at -20°C for up to 1 month. | [InChI]
1S/C5H5N5S/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H4,6,7,8,9,10,11) | [InChIKey]
WYWHKKSPHMUBEB-UHFFFAOYSA-N | [SMILES]
NC1=Nc2nc[nH]c2C(=S)N1 | [CAS DataBase Reference]
154-42-7(CAS DataBase Reference) | [EPA Substance Registry System]
154-42-7(EPA Substance) |
| Safety Data | Back Directory | [Hazard Codes ]
T,Xi | [Risk Statements ]
R25:Toxic if swallowed.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed . | [Safety Statements ]
S28:After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection .
S45:In case of accident or if you feel unwell, seek medical advice immediately (show label where possible) . | [RIDADR ]
UN 2811 6.1/PG 3
| [WGK Germany ]
3
| [RTECS ]
UP0740000
| [F ]
13 | [Hazard Note ]
Irritant | [HazardClass ]
6.1 | [PackingGroup ]
III | [HS Code ]
29335990 | [Storage Class]
6.1C - Combustible acute toxic Cat.3
toxic compounds or compounds which causing chronic effects | [Hazard Classifications]
Acute Tox. 3 Oral | [Hazardous Substances Data]
154-42-7(Hazardous Substances Data) | [Toxicity]
LD50 oral in mouse: 160mg/kg |
| Hazard Information | Back Directory | [General Description]
Odorless or almost odorless pale yellow crystalline powder. | [Reactivity Profile]
6-THIOGUANINE(154-42-7) is incompatible with strong oxidizing agents. . | [Air & Water Reactions]
This chemical may be sensitive to prolonged exposure to air. Insoluble in water. | [Fire Hazard]
Flash point data for this chemical are not available; however, 6-THIOGUANINE is probably combustible. | [Description]
An antineoplastic metabolic
antagonist that inhibits DNA synthesis by being metabolically
converted to 6-thioGMP. This inhibits purine biosynthesis at
multiple steps, and may be phosphorylated and incorporated
into DNA. In humans, it causes bone marrow depression and
gastrointestinal toxicity. Due to safety problems it is currently
less used as an antineoplastic agent but has some use as therapy for ulcerative colitis. | [Chemical Properties]
Crystalline, lyophilized, sterile, endot | [Originator]
Thioguanine,Burroughs-Wellcome,US,1966 | [Uses]
6-Thioguanine acts as an antineoplastic and purine antimetabolite. It is also useful as an inhibition of stimulated expression of TNF-related apoptosis-inducing ligand (TRAIL) protein. It is involved in the treatment of acute leukemias and Psoriasis. Further, it is used for the treatment of ulcerative colitis and autoimmune diseases. | [Uses]
antineoplastic, purine antimetabolite | [Uses]
The compound produced dose-dependent inhibition of stimulated expression of TRAIL protein | [Uses]
Thioguanine USP (Tabloid)is used to treat acute leukemia; chronic granulocytic leukemia. | [Application]
6-Thioguanine is a variant of guanine with hydrogen bonding at the N-7 of the purine ring. Its association with cytosine alters the dimension of the base stacking. 6-Thioguanine usage in treating inflammatory bowel disease (IBD) contributes to nodular regenerative hyperplasia (NRH) in the liver.
6-Thioguanine has been used:
to induce autophagy and apoptosis in colorectal cancer cell lines HCT116
as a selection marker in the mutation and survival assay in chinese hamster lung fibroblasts culture V79
as a selection marker in clonogenic Lung metastasis assay of 4T1-luc cells | [Definition]
ChEBI: A 2-aminopurine that is the 6-thiono derivative of 2-amino-1,9-dihydro-6H-purine. | [Indications]
6-Thioguanine is a purine analogue structurally related
to 6-mercaptopurine and azathioprine. Thioguanine interferes
with several enzymes required for de novo
purine synthesis, and its metabolites are incorporated
into DNA and RNA, further impeding nucleic acid synthesis.
The mechanism of action of thioguanine in psoriasis
is not clearly understood; it has been hypothesized
to affect the proliferation and trafficking of lymphocytes
as well as the proliferation of keratinocytes. | [Indications]
Thioguanine is an analogue of the natural purine guanine
in which a hydroxyl group has been replaced by a
sulfhydryl group in the 6-position. Two major mechanisms
of cytotoxicity have been proposed for 6-thioguanine:
(1) incorporation of the thio nucleotide analogue
into DNA or RNA and (2) feedback inhibition of purine
nucleotide synthesis.
The product of this reaction, 6-TGMP, can eventually
be converted to deoxy-6-thioguanosine-triphosphate
(dTGTP), which has been shown to be incorporated
into DNA. Resistance of human leukemia cells to
thioguanine has been correlated with decreased activity
of HGPRTase and to increased inactivation of the thio
nucleotides by alkaline phosphatase.
Thioguanine is slowly absorbed after oral administration;
parent drug levels are barely detectable, and
peak levels of metabolites occur only after 6 to 8 hours.
Total urinary excretion of metabolites in the first 24
hours is 24 to 46% of the administered dose.
Thioguanine is used primarily as part of a combined
induction of chemotherapy in acute myelogenous
leukemia.
Myelosuppression, with leukopenia and thrombocytopenia
appearing 7 to 10 days after treatment, and mild
nausea are the most common adverse effects. Liver toxicity
with jaundice has been reported in some patients but
appears to be less common than with mercaptopurine. | [Manufacturing Process]
A mixture of 2.7 grams of finely divided guanine, 10 grams of pulverized
phosphorus pentasulfide, 10 ml of pyridine and 100 ml of tetralin was heated
at 200°C with mechanical stirring for 5 hours. After cooling, the mixture was
filtered and the insoluble residue treated with 150 ml of water and 50 ml of
concentrated ammonium hydroxide. The ammoniacal solution was filtered,
heated to boiling and acidified with acetic acid. Upon cooling, 2-amino-6-
mercaptopurine precipitated as a dark yellow powder, according to US Patent
2,697,709. | [Brand name]
Tioguanine is INN and BAN. | [Therapeutic Function]
Cancer chemotherapy | [Biochem/physiol Actions]
Ribosylated and phosphorylated by the same pathway as natural purine bases; as the nucleotide, inhibits a variety of cellular processes involved in nucleic acid synthesis. Has a long history as an effective treatment of leukemia. | [Mechanism of action]
Absorption of orally administered 6-thioguanine is
slow and incomplete; only approximately 30% of the
oral dose is achieved in the plasma, peak levels being
reached after 8 hours. Thioguanine is extensively metabolized
prior to excretion. The elimination half-life is
on the order of 80 minutes. | [Clinical Use]
Although 6-thioguanine is chiefly used in chemotherapy
for acute myelocytic leukemia and other
marrow-based malignancies, lower doses are very effective
for moderate to severe psoriasis, particularly in patients who cannot tolerate alternative systemic agents
such as methotrexate and cyclosporine. | [Side effects]
Dose-related myelosuppression is the major adverse
effect produced by 6-thioguanine. Patients deficient in
thiopurine methyltransferase (TPMT), a cytosolic enzyme
required for metabolism of 6-thioguanine, are at
heightened risk. Other adverse effects include gastrointestinal
complaints and elevations of liver transaminases.
There have been rare reports of more serious hepatotoxicity,
including acute hepatitis, acute cholestasis,
and hepatic venoocclusive disease. | [Safety Profile]
Poison by ingestion andintraperitoneal routes. Human mutation data reported. Anexperimental teratogen. Other reproductive effects. Ahuman skin irritant. When heated to decomposition itemits very toxic fumes of SOx and NOx. | [Synthesis]
Thioguanine, 2-aminopurin-6-thiol (30.1.2.12), is made from 2,8-dichloro-
6-hydroxypurine (30.1.2.7), in which the second chlorine atom at C2 is replaced with an
amino group when reacted with ammonia, forming 2-amino-8-chloro-6-hydroxy-purine
(30.1.2.7), which is then reduced by hydrogen iodide to 2-aminopurin-6-ol (30.1.2.11).
Replacement of the hydroxyl group with a mercapto group at C6 is carried out by reacting
it with phosphorous pentasulfide, which forms thioguanine (30.1.2.12).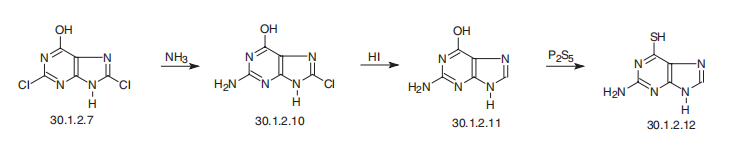
| [Veterinary Drugs and Treatments]
Thioguanine may be useful as adjunctive therapy for acute lymphocytic
or granulocytic leukemia in dogs or cats. | [Drug interactions]
Potentially hazardous interactions with other drugs
Antipsychotics: avoid concomitant use with
clozapine (increased risk of agranulocytosis). | [Metabolism]
Tioguanine undergoes extensive metabolism in the liver and other tissues to several active and inactive metabolites. Tioguanine is inactivated mainly by methylation to aminomethylthiopurine; small amounts are deaminated to thioxanthine, and may go on to be oxidised by xanthine oxidase to thiouric acid, but inactivation is essentially independent of xanthine oxidase and is not affected by inhibition of the enzyme. 24-46
% of the dose is excreted in the urine within 24 hours. It is excreted in the urine almost entirely as metabolites | [storage]
Room temperature | [Purification Methods]
It crystallises from H2O as needles. It has UV at 258 and 347nm (H2O, pH 1) and 242, 270 and 322nm max (H2O, pH 11). [Elion & Hitchings J Am Chem Soc 77 1676 1955, Fox et al. J Am Chem Soc 80 1669 1958.] It is an antineoplastic agent [Kataoka et al. Cancer Res 44 519 1984]. [Beilstein 26 III/IV 3926.] | [References]
1) Wang and Wang (2009), 6-thioguanine perturbs cytosine methylation at the CpG dinucleotide site by DNA methyltransferases in vitro and acts as a DNA demethylating agent in vivo; Biochemistry, 48 2290
2) Yuan et al. (2011), 6-thioguanine reactivates epigenetically silenced genes in acute lymphoblastic leukemia cells by facilitating proteasome-mediated degradation of DNMT1; Cancer Res., 71 1904
3) Bohon and de los Santos (2005), Effect of 6-thioguanine on the stability of duplex DNA; Nucleic Acid Res., 33 2880
4) Issaeva et al. (2010), 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance; Cancer Res., 70 6268 |
|
|